Microbiome Dysbiosis in Lichen Sclerosus: A Systematic Review
DOI:
https://doi.org/10.14740/jcgo1555Keywords:
Lichen sclerosus, Vulvar microbiome, Vaginal microbiome, Gut microbiome, Microbiome dysbiosis, Proteobacteria, Lactobacillus, FirmicutesAbstract
Background: Lichen sclerosus (LS) is a chronic, inflammatory skin condition primarily affecting the vulvar and perineal areas, often causing pain, pruritus, and scarring. While vulvar and vaginal microbiome composition is understood to contribute to genital health, their relationship with LS pathogenesis is unclear. Recent studies also suggest that gut microbiome imbalances may influence LS via systemic immune modulation. This systematic review aimed to characterize microbial alterations in the vulvar, vaginal, and gut microbiomes of LS patients and explore potential mechanisms linking dysbiosis to disease progression.
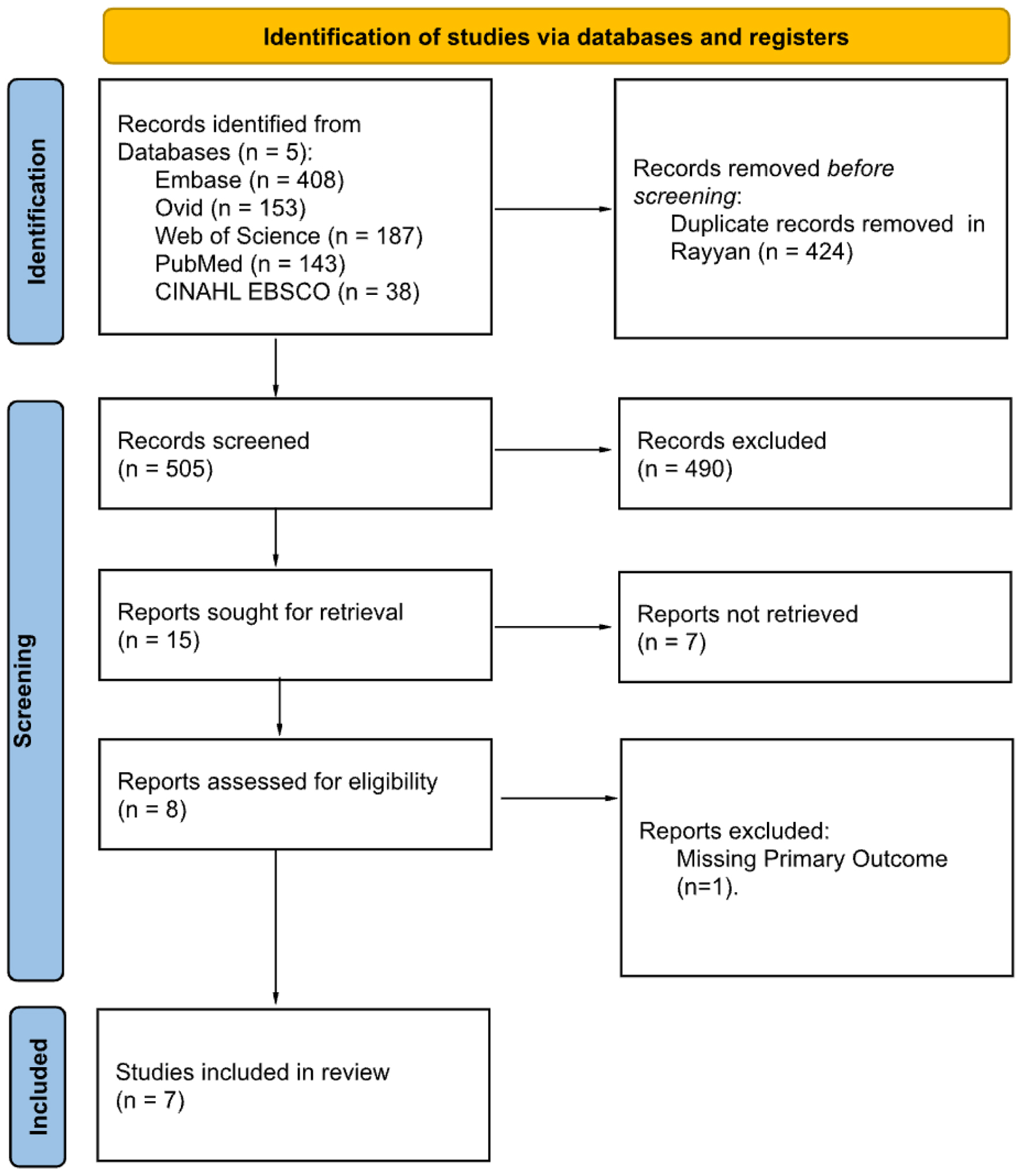
Methods: A systematic search was conducted using five databases: EMBASE, Medline via Ovid, Web of Science, PubMed, and CINAHL. Eligible studies included female patients diagnosed with LS and assessed the primary outcome of vulvar and vaginal microbiome composition. Gut microbiome data were considered a secondary outcome of interest. Following an initial double-blind screening, eight full-text articles were reviewed in the secondary screening, and seven articles were ultimately included in this review.
Results: Seven studies met the inclusion criteria. Across the vulvar, vaginal, and gut microbiomes, consistent patterns emerged, including reductions in protective taxa, such as Firmicutes and Lactobacillus, and increases in pro-inflammatory microbes, including Proteobacteria. Vulvar samples also showed higher abundances of Enterobacteriaceae, Peptoniphilus, and Campylobacter. Gut microbiome alterations included reduced short-chain fatty acid-producing bacteria, such as Firmicutes and Bacteroides, in addition to elevated Proteobacteria and Rikenellaceae. Alpha diversity findings were variable, and species-level changes were often inconsistent.
Conclusions: Microbial dysbiosis of the vulvar, vaginal, and gut microbiomes may contribute to the development of LS through chronic inflammation, compromised epithelial barriers, and disrupted immune function. While some alterations in the microbiomes were identified, discrepancies between the results of these studies highlight the need for larger, standardized studies to better understand the relationship between the microbiome and LS pathophysiology.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






