| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://jcgo.elmerpub.com |
Original Article
Volume 14, Number 4, December 2025, pages 149-154
A Randomized Controlled Trial Comparing Intracytoplasmic Sperm Injection and Conventional In Vitro Fertilization Outcomes in Patients With Advanced Maternal Age
Yasser Abdelbaseer Hashima, b, d , Mohamed S. Salemc
aRoyal College of Obstetricians and Gynaecologists, London, UK
bObstetrics and Gynaecology Department, Faculty of Medicine, Assiut University, Assiut, Egypt
cObstetrics and Gynaecology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt
dCorresponding Author: Yasser Abdelbaseer Hashim, Royal College of Obstetricians and Gynaecologists, London, UK
Manuscript submitted September 25, 2025, accepted December 3, 2025, published online December 11, 2025
Short title: IVF and ICSI Efficacy in Advanced Maternal Age
doi: https://doi.org/10.14740/jcgo1560
| Abstract | ▴Top |
Background: The most widely used fertilization treatment globally is intracytoplasmic sperm injection (ICSI), which is now indicated for a broader range of conditions. This study was designed to assess the outcomes of ICSI and conventional in vitro fertilization (c-IVF) specifically in advanced maternal age (AMA) patients.
Methods: This randomized controlled study included 70 women aged 39 - 44 years with a body mass index (BMI) of 18 - 35 kg/m2 and partners with normal semen parameters. After informed consent, each patient’s ovaries were randomized before stimulation: oocytes from one ovary (n = 250) underwent ICSI, and those from the other (n = 250) underwent conventional IVF.
Results: The average daily gonadotropin dose was 351.5 IU, with ovarian stimulation lasting about 9.7 days. Baseline follicle-stimulating hormone (FSH) and luteinizing hormone (LH) were 9.1 and 4.9 IU/L, respectively. Semen parameters included a volume of 2.1 mL, concentration of 72.5 million/mL, 59.7% motility, and 5.2% normal morphology. An average of 7.1 oocytes were retrieved per patient. In the ICSI and c-IVF groups, 71.2% and 72.4% of oocytes formed zygotes, with similar cleavage and top-quality embryo rates. Embryo transfers occurred in 54.8% (ICSI) and 57.6% (c-IVF) of cases. No substantial disparities were detected between the groups concerning their clinical and ongoing pregnancy rates.
Conclusions: In women aged 39 - 44 with normal sperm parameters, this study found no substantial variation in fertilization, embryo quality, or pregnancy rates between ICSI and c-IVF. Thus, ICSI offers no clear advantage over c-IVF in this population, suggesting that c-IVF remains a viable and effective option for patients with AMA.
Keywords: Advanced maternal age; In vitro fertilization; Intracytoplasmic sperm injection; Outcomes
| Introduction | ▴Top |
A combination of economic, social, and other factors has contributed to a rise in reproductive difficulties, exacerbated by the deferral of childbearing age, detrimental lifestyle habits, and psychological stress [1]. Infertility affects one in six couples more than once during their reproductive years worldwide [2].
The application of intracytoplasmic sperm injection (ICSI) has expanded markedly in recent decades, with a notable emphasis on addressing infertility that is not linked to male causes [3]. The initial purpose of its development was to enable fertilization in situations where severe male-derived infertility was present, and ICSI has evolved into the most frequently utilized method in assisted reproduction over the last three decades. Its use in Europe, for example, grew from 45% to 70%, although its frequency varies considerably across the globe [4]. There is a notable absence of clear evidence confirming the superiority of ICSI to conventional in vitro fertilization (c-IVF) for non-male factor infertility, despite the procedure’s increased prevalence [5].
Evidence suggests that ICSI can decrease the incidence of complete fertilization failure (FF); however, no such evidence exists to support its superiority in enhancing fertilization, blastocyst, or pregnancy rates in cases of non-male factor infertility when compared with c-IVF [6]. The live birth rates and clinical pregnancy do not notably differ between ICSI and c-IVF in cases of unexplained infertility [7].
Opinions remain divided on the use of ICSI as the preferred procedure for all couples requiring assisted reproductive technology (ART), particularly as a measure to prevent the up to 30% total FF rate observed during a primary c-IVF attempt [8]. Some investigations demonstrate that ICSI correlates with both a decreased risk of FF and an enhanced fertilization rate (FR) in comparison to c-IVF, whereas other studies have shown improved implantation, clinical pregnancy, and live birth rates with conventional insemination [9]. Due to the lack of a clear benefit, additional studies are required to assess ICSI’s role in an unexplained infertility population [10].
This investigation sought to evaluate and contrast the outcomes of ICSI and c-IVF among advanced maternal age (AMA) patients.
| Materials and Methods | ▴Top |
A total of 70 women, aged 39 - 44 years, participated in this randomized controlled trial. All had a body mass index (BMI) of 18 - 35 kg/m2 and partners with a normal sperm count (volume ≥ 1.5 mL, concentration ≥ 15 million/mL, motility ≥ 40%, and normal morphology ≥ 4%). Following the acquisition of informed written consent, patients were excluded if they had a prior cycle with an FR of less than 50%, used surgical sperm retrieval, had sperm or oocyte donation, or underwent preimplantation genetic testing.
The research was carried out in the Obstetrics and Gynaecology Department, Faculty of Medicine, Assiut University, Assiut, Egypt according to Helsinki Declaration.
Randomization and blindness
The study employed a web-based randomizer [11] to create a list for a 1:1 allocation, with individual patient codes sealed in opaque envelopes. Pre-ovarian stimulation, oocytes from one ovary (n = 250) were designated for ICSI, and those from the contralateral ovary (n = 250) were designated for c-IVF.
Stimulation protocol
On the third day of menses, gonadotropin treatment was initiated using recombinant follicle-stimulating hormone (FSH). The initiation of a combined regimen of a gonadotropin-releasing hormone (GnRH) antagonist (0.25 mg/day) and either recombinant FSH + luteinizing hormone (LH) or highly purified human menopausal gonadotropin was triggered when the leading follicle diameter reached 13 mm or estradiol (E2) levels exceeded 1,200 pmol/L. The progression of follicular growth and fluctuations in hormone levels were ascertained through routine ultrasound (US) imaging and blood analysis. Oocyte maturation was then instigated when a leading follicle measured 17 - 19 mm, followed by transvaginal oocyte retrieval 36 h after this event.
Ovaries were randomized per patient prior to ovarian stimulation. Oocytes retrieved from one side were designated for the ICSI group, and those from the contralateral side were allocated to the c-IVF group. Oocyte classification into the ICSI/IVF group was performed during the ovum pickup, based on the side of collection.
Embryo transfer (ET) was performed using the standard laboratory protocol by experienced embryologists. For all patients, embryos were cultured separately based on the ovary of origin. Embryos derived from the ovary allocated to ICSI and those derived from the ovary allocated to c-IVF were identified and recorded throughout culture.
For each patient, the number and quality of embryos available for transfer from each side were documented. When more than one embryo was suitable for transfer, the highest-quality embryos were selected irrespective of fertilization method. Thus, for every transfer, the number of embryos transferred from each group (ICSI vs. c-IVF) was documented.
Only one transfer procedure was performed per patient, and mixed transfers (embryos from both methods) were allowed when appropriate based on embryo quality. The total number of embryos transferred per patient and their origin were included in the analysis of clinical and ongoing pregnancy rates.
Embryo quality was classified in accordance with previously published scoring parameters [8]; top-quality embryos (TQE) were identified based on the following criteria: four to five blastomeres on day 2; at least seven blastomeres, equally sized blastomeres with ≤ 10% fragmentation, and the absence of multinucleation on day 3. Luteal support, involving daily administration of 90 mg of 8% vaginal progesterone gel, was commenced 1 day after oocyte pickup. Following a positive pregnancy test, ongoing gestation was verified by the presence of a gestational sac and fetal heart rate on an US conducted at 6 - 8 weeks.
The FR (the number of zygotes divided by the number of oocytes) served as the primary endpoint. Additional metrics included the quantity of cleavage-stage embryos, the number of TQE, the TQE rate per oocyte retrieved, and the rates of both clinical and ongoing pregnancy. Clinical pregnancy rate was determined by dividing the number of clinical pregnancies, identified by US visualization of one or more gestational sacs, by the number of ETs.
Sample size calculation
Based on prior FR (85.4% ICSI, 44.2% IVF) [12], sample size was calculated using G*Power 3.1.9.2 (Universitat Kiel, Germany) for 95% power and 0.05 α error. To account for dropouts, six extra cases were added, totaling 70 women.
Statistical analysis
For statistical analysis, SPSS v26 (IBM Inc., Chicago, IL, USA) was employed. Unpaired Student’s t-tests were used for comparisons of quantitative data (mean ± standard deviation (SD)) and for comparison before and after were compared by paired t-test, while the Mann-Whitney test was applied to non-parametric data between two groups and Wilcoxon test for comparison before and after, which are represented as median (interquartile range (IQR)). Categorical variables (%) were analyzed with the Chi-square or Fisher’s exact test, with statistical significance established at P ≤ 0.05.
| Results | ▴Top |
Of the 562 oocytes collected from 70 women screened for eligibility, 62 did not meet the criteria because of immature oocyte. The 70 women who proceeded provided 500 oocytes from each side, which were then randomly allocated to two equal groups, each consisting of 250 oocytes (Fig. 1).
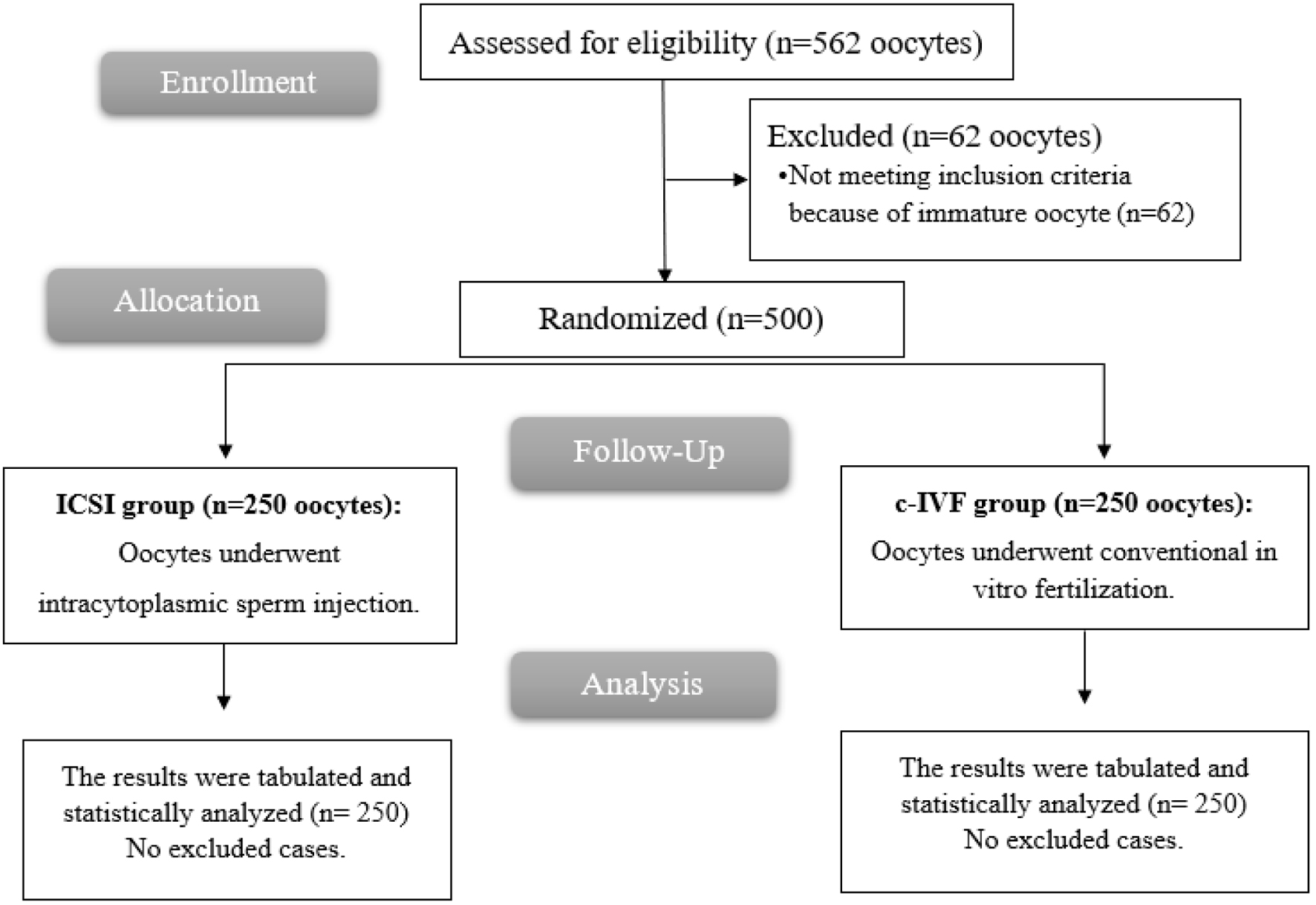 Click for large image | Figure 1. CONSORT flow chart of the enrolled patients. |
The mean value (± SD) of age was 41.3 (± 1.77) years. The mean value (± SD) of weight was 77.5 (± 9.89) kg. The mean value (± SD) of height was 163.4 (± 4.14) cm. The mean value (± SD) of BMI was 29.1 (± 3.83) kg/m2. Hypertension was present in nine (12.86%) patients, and diabetes was present in three (4.29%) patients (Table 1).
 Click to view | Table 1. Demographic Data and Comorbidities of the Studied Patients |
The median (IQR) of the daily dose of gonadotropins was 351.5 (231.25 - 431) IU. The mean value (± SD) of the length of stimulation was 9.7 (± 2.43) days. FSH levels, LH levels, estradiol levels, progesterone levels, and prolactin levels were significantly lower on the ET day than during ovarian stimulation (P < 0.001). The mean value (± SD) of anti-Müllerian hormone (AMH) levels was 2.1 (± 0.62) ng/mL during ovarian stimulation and on the ET day. Endometrial thickness was significantly higher on the ET day than during ovarian stimulation (P < 0.001). The mean value (± SD) of sperm volume was 2.1 (± 0.37) mL. The median (IQR) of sperm concentration was 72.5 (29 - 110.25) million/mL. The mean value (± SD) of motility was 59.7% (± 13.13%). The mean value (± SD) of normal morphology was 5.2% (± 0.74%). The mean value (± SD) of the mean number of oocytes per patient was 7.1 (± 2.94) (Table 2).
 Click to view | Table 2. Hormone Levels, Semen Parameters, and Total Oocyte Number of the Studied Patients |
There were no statistically significant differences between the ICSI and c-IVF groups regarding key embryological and clinical outcomes. The number of zygotes per retrieved oocytes and the count of cleavage-stage embryos were comparable between both groups (P = 0.766 and 0.696, respectively). Similarly, the number of TQEs, the TQE rate per oocyte retrieved, the number of embryos transferred, and number of blastocysts formed did not differ significantly. Clinical pregnancy and ongoing pregnancy rates were also similar between the two groups (P > 0.05). These findings indicate that both fertilization methods resulted in comparable embryological development and pregnancy outcome (Table 3).
 Click to view | Table 3. Outcomes of the Studied Groups |
| Discussion | ▴Top |
The ICSI, initially designed for severe male infertility, is now applied to non-male factors like AMA and poor ovarian response, despite limited evidence of improved outcomes [4, 13].
The FR demonstrated remarkable similarity between groups, with ICSI achieving 71.2% fertilization compared to 72.4% with c-IVF. The observed similarity aligned closely with findings from Fancsovits et al [4], who reported FR of 53.4% with ICSI versus 61.7% with c-IVF in a comparable population of women ≥ 40 years with non-severe male factor infertility. Similarly, Zhu et al [14] documented higher FR with ICSI (76.56%) compared to IVF (65.99%) in women > 35 years with ≤ 3 oocytes, yet this technical advantage failed to translate into improved clinical outcomes.
The comparable FRs in our study suggest that oocyte quality rather than fertilization method may be the primary determinant of successful fertilization in AMA patients with normal male partner parameters. Wu et al [15] showed that although overall FRs were similar between IVF and ICSI, the notably lower FR per mature oocyte in ICSI (75.0-77.8% vs. 91.5% in IVF) suggests potential oocyte damage from injection, reinforcing that ICSI offers no fertilization advantage without male factor indications.
The development of cleavage-stage embryos showed minimal variation between treatment groups, with 69.2% of ICSI oocytes and 70.8% of IVF oocytes progressing to the cleavage stage. This trend toward equivalent early embryonic development reinforces the concept that the fertilization method has a limited impact on subsequent embryo viability in non-male factor cases. The TQE rates per oocyte retrieved (63.6% ICSI versus 65.6% IVF) further support this conclusion, indicating that embryo quality assessment metrics remain unaffected by fertilization technique.
These findings aligned with multiple studies demonstrating comparable embryo quality outcomes. Garizi et al [13] reported superior embryo development parameters with IVF compared to ICSI in AMA patients with non-male factor infertility, while Jiesisibieke et al [16] noted that, despite increased FR with ICSI, c-IVF demonstrated superior implantation rates across different ovarian response categories. The mechanistic basis for these observations may relate to the invasive nature of ICSI, which bypasses natural sperm selection processes and may introduce cellular stress that affects subsequent embryonic development.
The ET rates demonstrated a slight trend favoring c-IVF, with 57.6% of cycles proceeding to transfer compared to 54.8% in the ICSI group. While this difference was not substantial, it suggests comparable clinical utility between approaches in terms of treatment accessibility.
The observed transfer rates aligned with expectations for AMA populations where embryo quality concerns may limit transfer opportunities. Zhu et al [14] reported lower cycle cancellation rates with ICSI (24.41%) compared to IVF (33.53%) in women > 35 years with ≤ 3 oocytes retrieved, yet this technical advantage did not translate into improved live birth outcomes. The similar transfer rates in our study suggest that in populations with adequate oocyte numbers, the potential benefits of reduced cycle cancellation with ICSI may be less pronounced.
Clinical pregnancy rates showed a trend favoring c-IVF (51.2%) over ICSI (47.2%), though the difference remained insignificant. This pattern extends to ongoing pregnancy rates, where c-IVF demonstrated 43.6% success compared to 41.2% with ICSI.
Fancsovits et al [4] reported higher clinical pregnancy rates with c-IVF (24.3%) compared to ICSI (19.0%) in a comparable population, with the difference nearing statistical significance, and observed notably poorer outcomes when AMA was combined with low oocyte numbers (18.5% for IVF vs. 4% for ICSI), findings that aligned with Garizi et al [13], who documented notably better pregnancy outcomes with IVF over ICSI in AMA patients without male factor infertility.
The consistency of these findings across multiple study populations suggests that the trend toward improved outcomes with c-IVF may reflect fundamental biological advantages of natural fertilization processes in non-male factor cases. Jiesisibieke et al [16] provided mechanistic insight, demonstrating that while ICSI increased FR, in categories of poor and suboptimal ovarian response, c-IVF cycles consistently demonstrated higher rates of implantation, clinical pregnancy, and live birth. Wu et al [15] further supported this concept, showing that in poor responders with single oocyte retrieval, c-IVF achieved superior live birth rates (11.5%) compared to ICSI in non-male factor cases (5%).
The small sample size constitutes a limitation of this study. Furthermore, the exclusion of severe male factor infertility restricts the generalizability of the results to wider AMA populations. The unique split-ovary design, while methodologically rigorous, may not reflect standard clinical practice where bilateral oocytes undergo uniform processing. Additionally, neonatal outcomes were not assessed, omitting potential long-term safety comparisons between techniques.
Conclusions
In AMA patients, with normal semen parameters, ICSI demonstrated no substantial advantage over c-IVF. FR (71.2% vs. 72.4%), TQE yields (63.6% vs. 65.6%), clinical pregnancy (47.2% vs. 51.2%), and ongoing pregnancy rates (41.2% vs. 43.6%) were comparable. These findings support reserving ICSI for male factor infertility and utilizing c-IVF as the primary insemination method for non-male factor AMA cases.
Acknowledgments
None to declare.
Financial Disclosure
No funding was received for conducting this study.
Conflict of Interest
The authors have no financial or proprietary interest in any material discussed in this article.
Informed Consent
An informed written consent was obtained from all patients.
Author Contributions
Both authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by YAH and MSS. The first draft of the manuscript was written by YAH and both authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Emokpae MA, Brown SI. Effects of lifestyle factors on fertility: practical recommendations for modification. Reprod Fertil. 2021;2(1):R13-R26.
doi pubmed - Rusanova NE. Infertility and fertility: demographic problems of assisted reproduction. Popul Econ. 2024;8(1):156-167.
- Glenn TL, Kotlyar AM, Seifer DB. The impact of intracytoplasmic sperm injection in non-male factor infertility-a critical review. J Clin Med. 2021;10(12):2616.
doi pubmed - Fancsovits P, Lehner A, Kaszas Z, Nemes A, Dudas B, Joo K, Murber A, et al. Intracytoplasmic sperm injection does not improve the outcome of IVF treatments in patients with advanced maternal age or low oocyte number: A randomized controlled trial. J Gynecol Obstet Hum Reprod. 2023;52(8):102625.
doi pubmed - Berntsen S, Zedeler A, Nohr B, Ronn Petersen M, Grondahl ML, Andersen LF, Lossl K, et al. IVF versus ICSI in patients without severe male factor infertility: a randomized clinical trial. Nat Med. 2025;31(6):1939-1948.
doi pubmed - Huang JX, Gao YQ, Chen XT, Han YQ, Song JY, Sun ZG. Impact of intracytoplasmic sperm injection in women with non-male factor infertility: A systematic review and meta-analysis. Front Reprod Health. 2022;4:1029381.
doi pubmed - Penzias A, Bendikson K, Falcone T, Hansen K, Hill M, Jindal S, Mersereau J. Evidence-based treatments for couples with unexplained infertility: a guideline. Fertil Steril. 2020;113(2):305-322.
doi pubmed - Haas J, Miller TE, Nahum R, Aizer A, Kirshenbaum M, Zilberberg E, Lebovitz O, et al. The role of ICSI vs. conventional IVF for patients with advanced maternal age-a randomized controlled trial. J Assist Reprod Genet. 2021;38(1):95-100.
doi pubmed - Balli M, Cecchele A, Pisaturo V, Makieva S, Carullo G, Somigliana E, Paffoni A, et al. Opportunities and limits of conventional IVF versus ICSI: It is time to come off the fence. J Clin Med. 2022;11(19):5722.
doi pubmed - Iwamoto A, Summers KM, Sparks A, Mancuso AC. Intracytoplasmic sperm injection versus conventional in vitro fertilization in unexplained infertility. F S Rep. 2024;5(3):263-271.
doi pubmed - http://www.randomizer.org.
- Gozlan I, Dor A, Farber B, Meirow D, Feinstein S, Levron J. Comparing intracytoplasmic sperm injection and in vitro fertilization in patients with single oocyte retrieval. Fertil Steril. 2007;87(3):515-518.
doi pubmed - Zare Garizi S, Sabagh Nezhad Yazd N, Tabibnejad N, Dehghani-Firouzabadi R. Pregnancy and neonatal outcomes of intracytoplasmic sperm injection versus in vitro fertilization in fresh cycles of women with advanced maternal age and nonmale factor infertility: A cross-sectional study. Int J Reprod Biomed. 2025;23(1):45-54.
doi pubmed - Zhu S, Li H, Lv Z, Liang X, Dong L, Tian D. Intracytoplasmic sperm injection compared with in vitro fertilisation in patients with non-male factor infertility with low oocyte retrieval: a single-centre, retrospective cohort study. BMJ Open. 2024;14(11):e080688.
doi pubmed - Wu CY, Huang TJ, Hwu YM, Kuo-Kuang Lee R, Lin MH. Comparison of clinical outcomes between conventional in vitro fertilization and intracytoplasmic sperm injection in poor responders with only single oocyte retrieved. Taiwan J Obstet Gynecol. 2023;62(1):55-58.
doi pubmed - Jiesisibieke D, Tian T, Zhu X, Fang S, Zhang N, Ma J, Xia Y, et al. Reproductive outcomes of conventional in vitro fertilization and intracytoplasmic sperm injection in patients with non-severe male infertility across poor and different sub-optimal ovarian response categories: a cohort study based on 30,352 fresh cycles from 2009-2019. Reprod Sci. 2024;31(5):1353-1362.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.