| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://jcgo.elmerpub.com |
Original Article
Volume 000, Number 000, August 2025, pages 000-000
Socioeconomic, Demographic, and Provider Determinants of Staged Versus Concomitant Surgery for Pelvic Organ Prolapse and Stress Urinary Incontinence
Jaime B. Longa, Krista Reaganb, Ryoko Satoc, g, Shunaha Kim-Fined, Kristen A. Gerjevice, Sarah Boyda, Olubiyi Aworunsec, Cara L. Grimesf
aDepartment of Obstetrics and Gynecology, Penn State College of Medicine, Hershey, PA, USA
bKaiser Permanente, Tacoma, WA, USA
cBoston Scientific, Marlborough, MA, USA
dDepartment of Obstetrics and Gynecology, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
eDepartment of Obstetrics and Gynecology, Creighton University School of Medicine, Omaha, NE, USA
fWestchester Medical Center and New York Medical College, Valhalla, NY, USA
gCorresponding Author: Ryoko Sato, Boston Scientific, Marlborough, MA 01752, USA
Manuscript submitted May 12, 2025, accepted July 9, 2025, published online August 7, 2025
Short title: Staged vs. Concomitant POP and SUI Surgery
doi: https://doi.org/10.14740/jcgo1521
| Abstract | ▴Top |
Background: While concomitant surgery for pelvic organ prolapse (POP) and stress urinary incontinence (SUI) may benefit patients, variability exists in practice. A better understanding of the decision-making process regarding the placement of mid-urethral slings (MUSs) in women undergoing POP surgery is crucial for achieving equitable surgical planning and patient counselling. This study assessed factors associated with staged vs. concomitant SUI surgery in patients undergoing POP surgery.
Methods: This retrospective cohort study used 100% Medicare Standard Analytical Files from 2016 to 2020. The sample population consisted of female patients surgically treated for POP with staged (within 24 months) or concomitant mid-urethral sling (MUS). Data were merged with the Agency for Healthcare Research and Quality (AHRQ) Social Determinants of Health Database. Multivariable logistic regression was used to assess independent associations between patient, location, and surgeon characteristics and the likelihood of staged versus concomitant procedures. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated.
Results: Among 40,842 patients with POP surgery, 96.7% (39,440) had concomitant, while 3.4% (1,402) had staged MUS. Most were aged 65 - 74 years (67.2%), Caucasian (91.7%), from urban areas (91.9%), and had Charlson Comorbidity Index ≤ 3 (82.3%). A higher likelihood of staged surgery was seen in White vs. other racial groups (example OR: 1.50 vs. Black, 95% CI: 1.03 - 2.18); in the South vs. other regions (example OR: 1.55 vs. Northeast, 95% CI: 1.26 - 1.91); and for obstetrics/gynecology vs. urologic surgeons (OR: 1.19, 95% CI: 1.02 - 1.40). Patients in counties with lower per capita income (OR: 1.02, 95% CI: 1.01 - 1.03), lower proportions of foreign-born (OR: 1.01, 95% CI: 1.01 - 1.05), limited English-speaking (OR: 1.03, 95% CI: 1.01 - 1.05), or uninsured (< 64 years of age; OR: 1.02, 95% CI: 1.00 - 1.03) households had higher likelihood of receiving staged surgery.
Conclusions: This study identified several demographic, socioeconomic, and surgeon-related factors associated with staged vs. concomitant MUS procedures among patients undergoing surgery for POP. The reasons for these differences are not fully understood and may reflect various underlying factors, including differences in communication, regional or specialty norms, patient choice, or other factors.
Keywords: Pelvic organ prolapse; Stress urinary incontinence; Mid-urethral sling; Staged surgery; Concomitant surgery; Sociodemographic
| Introduction | ▴Top |
Stress urinary incontinence (SUI) and pelvic organ prolapse (POP) are highly prevalent pelvic floor disorders [1-8] that are significant public health issues affecting women’s psychological state, quality of life, and sexual function [5]. Because SUI and POP frequently coexist, the potential for de novo SUI following POP treatment is commonly taken into consideration during surgical planning [9-11]. Mid-urethral slings (MUS) have traditionally been the standard of care and most common surgical procedure for SUI [12]. MUS may be placed at the time of POP surgery for treatment of current SUI, as well as prevention of de novo SUI [10, 11]. While concomitant surgery for POP and SUI may benefit patients, the published literature suggests variation amongst practitioners [13-20]. Clinical outcomes such as sling revision or removal are important factors in shared decision making, and existing literature suggests potential benefit of concomitant surgery in this regard [10, 11, 21, 22]. However, some SUI cases resolve post-POP surgery without additional anti-incontinence procedures, and thus staging surgeries could prevent some patients from receiving unnecessary SUI surgeries, as well as the potential for related risks such as short-term voiding dysfunction and morbidity which are inherent in MUS surgeries [23-28].
The decision-making process regarding the placement of MUS in women undergoing POP surgery is thus multifaceted and potentially influenced by a variety of non-clinical factors including patient demographics, the quality of patient-surgeon communication in shared decision-making, surgeon specialty, cultural norms, patient or surgeon preference, and geographic location. Understanding the presence of these factors is crucial for achieving equitable surgical planning and patient counselling. This study aims to gain a better understanding of the patient-, location-, and surgeon-level factors associated with the utilization of staged versus concomitant SUI surgery among women undergoing POP surgery in the United States.
| Materials and Methods | ▴Top |
Data source
This retrospective cohort study used claims data from the US Centers for Medicare and Medicaid 100% Standard Analytic Files (SAF). This database includes traditional Medicare fee-for-service beneficiaries in the United States, the majority of whom are 65 and over. Medicare claims in the 100% SAF file capture all visits, except office and ambulatory surgical center (ASC) visits, and provide detailed claims-level information, including diagnosis and procedure codes (International Classification of Diseases, Ninth and 10th Revision, Clinical Modification (ICD-9-CM/ICD-10-CM)), Medicare Severity Diagnosis Related Group (MS-DRG), dates of service, hospital provider number, and beneficiary demographic information. After enrollment in Medicare, beneficiaries remained enrolled until death, allowing for robust longitudinal analysis within SAF files. As with all administrative claims data, the Medicare files used in this study were collected for billing and administrative purposes rather than research, which may introduce misclassification or limit the availability of clinical details.
The Medicare data were merged with the Agency for Healthcare Research and Quality (AHRQ) Social Determinants of Health (SDOH) Database. This database includes variables corresponding to five key SDOH domains: social context (e.g., age, race/ethnicity, veteran status), economic context (e.g., income, unemployment rate), education, physical infrastructure (e.g., housing, crime, transportation), and healthcare context (e.g., health insurance). The AHRQ SDOH data were linked to the Medicare data by geographic location (i.e., counties the patients resided in).
The study was determined to be exempt from Institutional Review Board (IRB) approval because it used data from an anonymous, deidentified administrative claims database that complies with the Health Insurance Portability and Accountability Act of 1996.
Patient population
The Medicare 100% SAF data were used to identify female patients aged ≥ 65 years and older who underwent surgical treatment for POP between 2016 and 2020 and with an MUS procedure (Current Procedural Terminology (CPT) code 57288). The study followed the previously published protocol for identifying staged vs. concomitant MUS/POP surgeries [21] and included patients who had concomitant MUS procedure, as well as those who had a staged procedure. Patients were classified as having a concomitant MUS procedure if it was performed during the same encounter as the POP surgery, or as having a staged procedure if the MUS was performed within 24 months following the POP surgery. Eligible patients were required to have 3-year continuous enrollment prior to their index POP surgery. Exclusion criteria included men, MUS placement within 1 year prior to the index POP surgery date, fascial harvesting, and repeat sling procedures. Patients who underwent fascial harvesting sling procedures, representing a very small subset, were excluded to maintain sample consistency. Similarly, urethral bulking was also not considered for the current analysis given its limited recommended or studied use for occult SUI. It is important to note that the study did not require a documented preoperative diagnosis of SUI, given the potential for undercoding in the claims data used. Figure 1 illustrates the study design and patient selection for the concomitant (Fig. 1a) and staged (Fig. 1b) MUS cohorts.
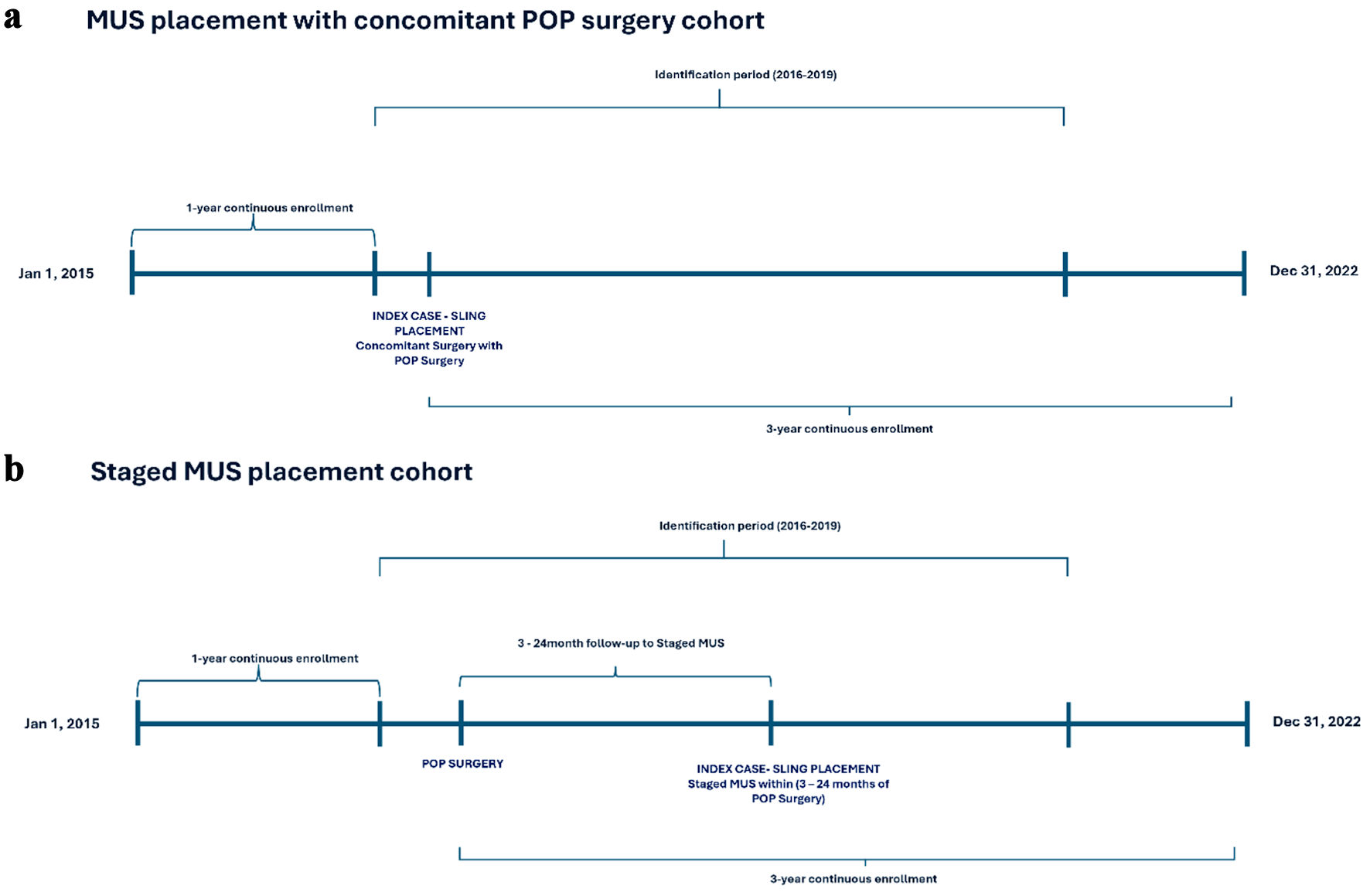 Click for large image | Figure 1. Study design and cohort selection. MUS: mid-urethral sling; POP: pelvic organ prolapse. |
Study measures
The primary outcome of interest was MUS concomitant with POP surgery or staged MUS within 24 months after the index POP surgery. Patient demographic and clinical characteristics included age, race, geographic region, urbanization, and Charlson Comorbidity Index (CCI) [29-31]. CCI comorbidities were used to assess patients’ baseline comorbidity scores. County-level characteristics derived from the AHRQ SDOH 2019 database included the percentage of foreign-born households, the percentage of limited English-speaking households, the Gini Index of income inequality, the median household income, the per capita income (units of $1,000), the percentage of population with any postsecondary education, the percentage of population with no health insurance coverage, the percentage of population < 64 years of age with no health insurance coverage, the total number of obstetric gynecologists per 1,000 population, and the median and mean distance in miles to the nearest obstetrics department. The Gini index measures the extent to which the distribution of income or consumption among individuals or households within an economy deviates from a perfectly equal distribution [32]. The index varies from 0 to 1, with higher values indicating greater inequality [32]. The specialty of surgeons performing POP procedures (i.e., urology, obstetrics/gynecology (OBGYN), or other) was used to characterize surgeon characteristics.
Statistical analyses
Continuous patient and provider characteristic variables were presented as means and standard deviations, while categorical patient and provider characteristics were reported as frequencies and percentages. Patients were categorized into two groups: staged or concomitant MUS cohorts. Chi-squared tests were used to test for statistical differences in categorical variables and t-tests for continuous variables. Following bivariate analyses, multivariable logistic regression was used to assess independent associations between patient, location, and surgeon characteristics and the likelihood of staged versus concomitant procedures. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. All analyses were performed using the Instant Health Data (IHD) software (Panalgo, Boston MA, USA) and R, version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria) at a priori significance of 0.05.
| Results | ▴Top |
Among 40,842 patients who had POP surgery between 2016 and 2020, 96.7% (n = 39,440) had a concomitant MUS procedure, while 3.4% (n = 1,402) had a staged MUS procedure.
Patient demographic and clinical characteristics
The majority of patients were aged between 65 and 74 years (67.2%), Caucasian (91.7%), resided in urban areas (91.9%), and had a CCI score ≤ 3 (82.3%) (Table 1). The concomitant and staged cohorts exhibited differences in terms of age category (P = 0.022), race (P = 0.003), geographic region (P < 0.001), provider specialty for the POP (P = 0.024), and provider specialty for the MUS (P < 0.001) (Table 1). In particular, compared to patients undergoing concomitant procedures, greater proportions of patients undergoing staged procedures were aged between 70 and 79 (55.7% vs. 51.6%), Caucasian (94.4% vs. 91.6%), lived in the Southern census region of the United States (46.0% vs. 39.9%), had an OBGYN surgeon for the POP repair (81.0% vs. 80.1%) and urologic surgeon for the MUS (19.7% vs. 15.7%) (Table 1).
 Click to view | Table 1. Baseline Demographic and Clinical Characteristics of US Medicare Patients With POP and MUS |
County-level characteristics
Counties where patients underwent staged procedures had lower proportions of households with foreign-born residents (10.2% vs. 11.6%; P < 0.001), limited English-speaking individuals (3.1% vs. 3.6%; P < 0.001), and residents with post-secondary education (59.5% vs. 60.3%; P = 0.003) compared to counties where patients underwent concomitant procedures (Table 2). In addition, counties with staged procedures had higher proportions of households with individuals < 64 years of age who were uninsured (10.6% vs. 10.3%; P = 0.038).
 Click to view | Table 2. County-Level AHRQ SDOH Characteristics of US Medicare Patients With POP and MUS |
Counties where patients underwent staged procedures also had a lower mean Gini Index (0.454 vs. 0.457; P = 0.007), lower median household income ($USD61,906 vs. $USD64,298; P < 0.001), and lower per-capita income ($USD32,296 vs. $USD33,448; P < 0.001) compared to counties with concomitant procedures. There were no significant differences in the density of OBGYN surgeons or the distance to the nearest obstetrics department between the counties of patients with staged and concomitant MUS (Table 2).
Multivariable analyses of factors associated with staged versus concomitant surgery
The multivariable analyses found that White race was associated with higher likelihood of receiving staged vs. concomitant surgery than other races: vs. Black (OR: 1.497, 95% CI: 1.029 - 2.177), Asian (OR: 2.585, 95% CI: 1.065 - 6.270), and Hispanic (OR: 2.028, 95% CI: 1.140 - 3.609) (Table 3 and Supplementary Material 1, jcgo.elmerpub.com). The outcome variable was reversed from staged to concomitant procedure to allow for alternative interpretation of odds ratios (ORs) (Supplementary Material 1, jcgo.elmerpub.com). Patients in the South were more likely to receive staged vs. concomitant surgery than other regions: vs. Northeast (OR: 1.550, 95% CI: 1.256 - 1.914), Midwest (OR: 1.228, 95% CI: 1.046 - 1.440), and West (OR: 1.289, 95% CI: 1.095 - 1.517). OBGYN vs. urology (OR: 01.193, 95% CI: 1.018 - 1.399) provider was also associated with higher likelihood of receiving staged vs. concomitant surgery (Table 3). Patients living in counties with lower per capita income (OR: 1.021, 95% CI: 1.009 - 1.034) and those living in counties with lower proportions of foreign-born (OR: 1.014, 95% CI: 1.005 - 1.054), limited English-speaking (OR: 1.032, 95% CI: 1.011 - 1.054), or uninsured (individuals < 64 years of age; OR: 1.016, 95% CI: 1.001 - 1.031) households were more likely to receive staged surgery. Urban setting, the proportion of households with postsecondary education (age ≥ 25), OBGYN density, and distance to the nearest obstetrics department were not significantly associated with receiving concomitant or staged SUI surgery (Table 3).
 Click to view | Table 3. Multivariable Analysis of Factors Associated With Staged Versus Concomitant Surgery |
| Discussion | ▴Top |
POP and SUI significantly impact women’s quality of life [4, 5]. The literature suggests a variety of treatment algorithms for POP and SUI, with varying clinical application of treatment guidelines among POP and SUI providers [13-20]. In this study, we evaluated the socioeconomic factors associated with the utilization of staged versus concomitant SUI surgery among patients undergoing POP surgery. Understanding if and how these factors are associated with this decision could enhance the surgical planning of POP and SUI, potentially improving shared decision-making and outcomes.
This study, based on over 40,000 Medicare patients, found that 96.7% underwent concomitant MUS procedures, while 3.4% had staged MUS procedures. These findings are consistent with rates observed in a prior US commercial claims database analysis between 2006 and 2014, which also found that 3.4% of MUS procedures were staged vs. concomitant [21]. Although concomitant MUS placement during POP repair is common and often used to address existing SUI, preoperative SUI may resolve in about one-third of women after prolapse surgery without additional concomitant incontinence procedures [28]. Similarly, while concomitant MUS placement aims to prevent de novo SUI, not all patients develop SUI following POP repair [13]. Therefore, while concomitant surgery for POP and SUI may benefit some patients, staging POP and SUI surgeries in appropriate patients may prevent unnecessary SUI procedures and complications in other patients [23-28]. Employing shared decision making to discuss the complicated option of staging or including a SUI surgery with POP surgery can be challenging, even amongst other medical professionals who speak the same language. Our finding that fewer than 4% of patients required subsequent sling surgery suggests that clinicians’ preoperative judgment in selecting MUS placement is generally accurate [28]. Additionally, previous studies have shown that the need for sling revision remains low, particularly in concomitant SUI and POP procedures [21], further supporting the effectiveness of initial surgical decision-making.
The multivariable analyses found that White women, residence in the Southern United States, and POP surgery performed by an OBGYN-trained surgeon were associated with a higher likelihood of receiving staged SUI surgery. Similarly, living in counties with lower per capita income and with lower proportions of households with foreign-born, limited English-speaking, or uninsured (< 64 years of age) individuals were associated with a higher likelihood of receiving staged surgery.
These findings suggest that treatment decisions for POP and SUI are not solely driven by clinical factors but may also be influenced by a complex interplay of socioeconomic factors, healthcare access, regional practice patterns, and provider specialty. White women, patients in the Southern United States, and those treated by OBGYN-trained surgeons were more likely to undergo staged rather than concomitant MUS placement, potentially reflecting differences in provider training, regional practice patterns, and patient preferences. Additionally, patients from counties with fewer foreign-born and fewer limited English-speaking individuals were more likely to undergo staged surgery. These differences may result from variations in surgeon practices by specialty or region, healthcare access, physician-patient communication, or other factors rather than purely clinical considerations. Because there is no universal algorithm that is “right” or “wrong” for making these shared decisions, it is unclear whether these differences reflect differences in care, counselling, or other influencing factors. Future studies should explore how socioeconomic and surgeon characteristics may impact decisions regarding concomitant vs. staged MUS procedures [13, 23-28].
A key strength of this study is the use of Medicare data, which provides meaningful pragmatic clinical and economic data for a large patient population. Administrative claims studies leverage data originating from clinical practice and may provide a more accurate representation of real-world conditions than clinical trials. This evidence may be useful in assisting consumers, clinicians, and policymakers in making informed decisions [33, 34]. Additionally, the inclusion of numerous covariates in the logistic regression model, including baseline patient characteristics, socioeconomic, and provider characteristics, enhances the robustness of our findings. The use of logistic regression to adjust for differences in baseline characteristics enabled the elucidation of independent patient, location, and surgeon characteristics associated with the likelihood of staged versus concomitant procedures. The integration of AHRQ SDOH data allows for a comprehensive examination of the relationships between health, SDOH, and healthcare.
However, there are limitations to this study. As it relies on administrative data, it is important to acknowledge that billing data are not designed specifically for research. Consequently, the absence of detailed preoperative clinical variables (such a preoperative diagnosis of stress incontinence), which may have been correlated with the study outcome, introduces a potential confounding issue. As such, the study does not make a clear assessment of the role of explanatory variables. Billing data may also be subject to clerical inaccuracies, recording biases due to financial incentives, and temporal changes in billing codes [35, 36]. Retrospective study design only informs the correlation but not the causal link. Additionally, the racial variable in Medicare data merges race (e.g., White, Black, Asian) with ethnicity (Hispanic vs. non-Hispanic), preventing the distinction between the two and limiting the interpretability of the results. Also, findings from the Medicare and AHRQ SDOH databases may not be generalizable to all patients in the United States or to patients in other countries. Given that all patients were > 65 years old, the findings are not generalizable to younger patients undergoing prolapse and incontinence surgery. Furthermore, key information, such as individual English proficiency, is only available at the county level, limiting the ability to accurately interpret the individual impact on the observed associations. Finally, surgeon characteristics could not be further classified into sub-specialty, specifically urogynecology-trained surgeons either with a gynecology or urology background. Thus, we cannot make definitive conclusions about differences between OBGYN specialist and urogynecology sub-specialist practices and the impact on staged sling procedures. Because the analysis lacks key clinical data such as symptom severity, urodynamic findings, or patient-reported outcomes, the clinical relevance and granularity of the observed associations are inherently limited.
This study identified demographic, socioeconomic, and surgeon-related factors associated with staged versus concomitant MUS procedures among patients undergoing surgery for POP. The reasons for these differences are not fully understood and may reflect various underlying factors, including differences in communication, regional or specialty norms, patient choice, or other factors. Future research should further investigate how these socioeconomic and surgeon characteristics impact the complex decision to undergo a concomitant vs. staged MUS procedure.
| Supplementary Material | ▴Top |
Suppl 1. Multivariable analysis of factors associated with concomitant versus staged surgery.
Acknowledgments
The authors thank Natalie Edwards of Health Services Consulting Corporation, Boxborough, MA, USA for editorial assistance with the manuscript.
Financial Disclosure
This study was funded by Boston Scientific, Inc.
Conflict of Interest
RS and OA are employees of Boston Scientific, Inc. Shunaha Kim-Fine is on the speaker’s bureau for Lupin Pharma Inc. and Duschenay Inc. The remaining authors reported no disclosures.
Informed Consent
Not applicable.
Author Contributions
JBL, KR, RS, SKF, KAG, SB, OA, and CLG participated in the conception and design of the study, the data collection, analysis, and interpretation of the data, manuscript writing and, in the review, and final approval of the manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
AHRQ: Agency for Healthcare Research and Quality; ASC: ambulatory surgical center; CCI: Charlson Comorbidity Index; CI: confidence interval; CPT: Current Procedural Terminology; POP: pelvic organ prolapse; ICD-9-CM/ICD-10-CM: International Classification of Diseases, Ninth and 10th Revision, Clinical Modification; IHD: Instant Health Data; IRB: Institutional Review Board; MS-DRG: Medicare Severity Diagnosis Related Group; MUS: mid-urethral sling; OBGYN: obstetrics/gynecology; OR: odds ratio; SAF: Standard Analytic Files; SDOH: Social Determinants of Health; SUI: stress urinary incontinence
| References | ▴Top |
- Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, Spino C, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311-1316.
doi pubmed - Wu JM, Vaughan CP, Goode PS, Redden DT, Burgio KL, Richter HE, Markland AD. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet Gynecol. 2014;123(1):141-148.
doi pubmed - Barber MD, Maher C. Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J. 2013;24(11):1783-1790.
doi pubmed - Wang B, Chen Y, Zhu X, Wang T, Li M, Huang Y, Xue L, et al. Global burden and trends of pelvic organ prolapse associated with aging women: An observational trend study from 1990 to 2019. Front Public Health. 2022;10:975829.
doi pubmed - Ozengin N, Cankaya H, Duygu E, Uysal MF, Bakar Y. The effect of pelvic organ prolapse type on sexual function, muscle strength, and pelvic floor symptoms in women: A retrospective study. Turk J Obstet Gynecol. 2017;14(2):121-127.
doi pubmed - Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187(1):116-126.
doi pubmed - Lugo T, Leslie SW, Mikes BA, Riggs J. Stress urinary incontinence. In: StatPearls. Treasure Island (FL) ineligible companies. 2025.
pubmed - Aboseif C, Liu P. Pelvic organ prolapse. StatPearls. Treasure Island (FL): StatPearls Publishing. StatPearls Publishing LLC.; 2024.
- Patel PD, Amrute KV, Badlani GH. Pelvic organ prolapse and stress urinary incontinence: A review of etiological factors. Indian J Urol. 2007;23(2):135-141.
doi pubmed - Brubaker L, Cundiff GW, Fine P, Nygaard I, Richter HE, Visco AG, Zyczynski H, et al. Abdominal sacrocolpopexy with Burch colposuspension to reduce urinary stress incontinence. N Engl J Med. 2006;354(15):1557-1566.
doi pubmed - Wei JT, Nygaard I, Richter HE, Nager CW, Barber MD, Kenton K, Amundsen CL, et al. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366(25):2358-2367.
doi pubmed - Nager C, Tulikangas P, Miller D, Rovner E, Goldman H. Position statement on mesh midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2014;20(3):123-125.
doi pubmed - Baessler K, Christmann-Schmid C, Maher C, Haya N, Crawford TJ, Brown J. Surgery for women with pelvic organ prolapse with or without stress urinary incontinence. Cochrane Database Syst Rev. 2018;8(8):CD013108.
doi pubmed - Pelvic organ prolapse: ACOG practice bulletin, Number 214. Obstet Gynecol. 2019;134(5):e126-e142.
doi pubmed - Ontario H. Vaginal pessaries for pelvic organ prolapse or stress urinary incontinence: a health technology assessment. Ont Health Technol Assess Ser. 2021;21(3):1-155.
pubmed - Raju R, Linder BJ. Evaluation and management of pelvic organ prolapse. Mayo Clin Proc. 2021;96(12):3122-3129.
doi pubmed - El-Nashar SA, Singh R, Chen AH. Pelvic organ prolapse: overview, diagnosis and management. Journal of Gynecologic Surgery. 2022;39(1):3-11.
- Lau HH, Davila GW, Chen YY, Sartori MGF, Jarmy-Di Bella ZIK, Tsai JM, Liu YM, et al. FIGO recommendations: Use of midurethral slings for the treatment of stress urinary incontinence. Int J Gynaecol Obstet. 2023;161(2):367-385.
doi pubmed - Pizzoferrato AC, Thuillier C, Venara A, Bornsztein N, Bouquet S, Cayrac M, Cornillet-Bernard M, et al. Management of female pelvic organ prolapse-Summary of the 2021 HAS guidelines. J Gynecol Obstet Hum Reprod. 2023;52(3):102535.
doi pubmed - Wang CN, Chung DE. Etiology, diagnosis, and management of pelvic organ prolapse: overview. In: Martins FE, Holm HV, Sandhu JS, McCammon KA, editors. Female genitourinary and pelvic floor reconstruction. Cham: Springer International Publishing; 2023. p. 507-518.
- Boyd SS, Long JB, Agbese E, Leslie D. Incidence of midurethral sling revision or removal by its timing with prolapse surgery. Female Pelvic Med Reconstr Surg. 2022;28(6):379-384.
doi pubmed - Sharif F, Mahmud F, Suman S, Cheng AL, Shepherd JP, Sutkin G. Risk factors for returning to the operating room for a second surgery after midurethral sling for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2020;26(7):443-446.
doi pubmed - Colombo M, Vitobello D, Proietti F, Milani R. Randomised comparison of Burch colposuspension versus anterior colporrhaphy in women with stress urinary incontinence and anterior vaginal wall prolapse. BJOG. 2000;107(4):544-551.
doi pubmed - Costantini E, Lazzeri M, Bini V, Del Zingaro M, Zucchi A, Porena M. Burch colposuspension does not provide any additional benefit to pelvic organ prolapse repair in patients with urinary incontinence: a randomized surgical trial. J Urol. 2008;180(3):1007-1012.
doi pubmed - Borstad E, Abdelnoor M, Staff AC, Kulseng-Hanssen S. Surgical strategies for women with pelvic organ prolapse and urinary stress incontinence. Int Urogynecol J. 2010;21(2):179-186.
doi pubmed - Shepherd JP, Alperin M, Meyn LA, Frankman EA, Zyczynski HM. Now or later…Does timing of a midurethral sling in relation to transvaginal prolapse repair affect continence outcomes at 1 year? Female Pelvic Med Reconstr Surg. 2010;16(5):299-303.
doi pubmed - van der Ploeg JM, Oude Rengerink K, van der Steen A, van Leeuwen JH, Stekelenburg J, Bongers MY, Weemhoff M, et al. Transvaginal prolapse repair with or without the addition of a midurethral sling in women with genital prolapse and stress urinary incontinence: a randomised trial. BJOG. 2015;122(7):1022-1030.
doi pubmed - Giugale LE, Carter-Brooks CM, Ross JH, Shepherd JP, Zyczynski HM. Outcomes of a Staged Midurethral Sling Strategy for Stress Incontinence and Pelvic Organ Prolapse. Obstet Gynecol. 2019;134(4):736-744.
doi pubmed - Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-682.
doi pubmed - Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
doi pubmed - Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139.
doi pubmed - Truman BI, Smith KC, Roy K, Chen Z, Moonesinghe R, Zhu J, Crawford CG, et al. Rationale for regular reporting on health disparities and inequalities - United States. MMWR Suppl. 2011;60(1):3-10.
pubmed - Dreyer NA, Schneeweiss S, McNeil BJ, Berger ML, Walker AM, Ollendorf DA, Gliklich RE, et al. GRACE principles: recognizing high-quality observational studies of comparative effectiveness. Am J Manag Care. 2010;16(6):467-471.
pubmed - Schneeweiss S, Gagne JJ, Glynn RJ, Ruhl M, Rassen JA. Assessing the comparative effectiveness of newly marketed medications: methodological challenges and implications for drug development. Clin Pharmacol Ther. 2011;90(6):777-790.
doi pubmed - Patel AA, Singh K, Nunley RM, Minhas SV. Administrative databases in orthopaedic research: pearls and pitfalls of big data. J Am Acad Orthop Surg. 2016;24(3):172-179.
doi pubmed - Bohl DD, Singh K, Grauer JN. Nationwide databases in orthopaedic surgery research. J Am Acad Orthop Surg. 2016;24(10):673-682.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.