| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 000, Number 000, December 2024, pages 000-000
Spontaneous Subarachnoid Hemorrhage After Cesarean Section
Jijisha Alia, e , Dora Dimarucuta, Rida Maryumb, Shriganesh Shankarrao Patilc, Tejal Desaid
aDepartment of Obstetrics and Gynecology, Mediclinic Welcare Hospital, Dubai, United Arab Emirates
bCollege of Medicine, Mohammed Bin Rashid University, Dubai, United Arab Emirates
cDepartment of Radiology, Mediclinic Welcare Hospital, Dubai, United Arab Emirates
dDepartment of Anesthesia, Mediclinic Welcare Hospital, Dubai, United Arab Emirates
eCorresponding Author: Jijisha Ali, Department of Obstetrics and Gynecology, Mediclinic Welcare Hospital, Dubai, United Arab Emirates
Manuscript submitted October 15, 2024, accepted November 29, 2024, published online December 21, 2024
Short title: SAH After Cesarean Section
doi: https://doi.org/10.14740/jcgo1004
| Abstract | ▴Top |
Subarachnoid hemorrhage (SAH) is a medical emergency commonly caused by the rupture of cerebral aneurysms, hypertensive disorders, arteriovenous malformations (AVMs), trauma, or spontaneous events, etc. In the case of postpartum women, SAH is especially rare and can be easily misdiagnosed due to its unexpected nature in the context of recent delivery. This abstract emphasizes that SAH following a cesarean section (C-section) is very rare. This highlights the importance of the case, as obstetricians and neurologists must be aware of such complications even though they are uncommon and present significant clinical challenges. While the majority of these events are unrelated to pregnancy, the physiological changes during pregnancy and the postpartum period may increase the risk of vascular rupture. This case report documents the rare occurrence of spontaneous SAH in a 28-year-old female on the fourth postoperative day following an emergency C-section under epidural analgesia, with no prior neurological symptoms or antenatal complications. Early recognition, multidisciplinary management, and prompt neurosurgical intervention were key to the patient’s recovery. This report highlights the importance of vigilance for neurological complications in the postpartum period. The purpose of this case report is to highlight the diagnostic and management challenges of spontaneous SAH in the postpartum period, a condition that is exceedingly rare but potentially life-threatening. By documenting this case, we aim to contribute to the understanding of postpartum SAH, emphasizing the need for heightened clinical vigilance, timely diagnosis, and multidisciplinary management in ensuring favorable outcomes.
Keywords: Subarachnoid hemorrhage; Cesarean section; Postpartum; Aneurysm; Obstetric complications
| Introduction | ▴Top |
Spontaneous subarachnoid hemorrhage (SAH) is an uncommon but serious condition often presenting with severe headache [1]. The primary causes of SAH are intracranial aneurysms, accounting for 51-80% of cases, and hypertensive disorders at 15%. Arteriovenous malformations (AVMs) are responsible for 5-10% of cases. Less frequent causes include intracranial infections, tumors, coagulopathies, and blood disorders [2]. In 5-10% of cases, the cause remains unknown [3]. During pregnancy, the risk of spontaneous SAH increases fivefold, primarily due to a higher likelihood of aneurysm rupture and AVM rebleeding during this period [4]. It is a potentially life-threatening condition, most commonly associated with aneurysmal rupture. However, its occurrence in the postpartum period, particularly after cesarean section (C-section) is exceptionally uncommon and presents unique diagnostic and therapeutic challenges. SAH is a life-threatening condition typically marked by a sudden, intense “thunderclap” headache, often referred to as the worst headache a person has ever experienced. SAH occurring after spinal or epidural anesthesia is extremely uncommon, yet it represents a serious and rare complication in these situations [5]. SAH occurs in roughly 5.8 per 100,000 deliveries among women between the ages of 15 and 44 [6].
Ethical approval for the publication of this case report was obtained from the Institutional Review Board (IRB), ensuring adherence to ethical guidelines for patient confidentiality and informed consent.
| Case Report | ▴Top |
Investigations
We report the case of a 28-year-old female (para 0, aborta 1) with an uneventful antenatal course. Her previous pregnancy had resulted in a blighted ovum, which was managed with dilation and evacuation. During the current pregnancy, she had no complications such as gestational hypertension or preeclampsia. Her medical history was only significant for asthma. The patient was admitted at 39 weeks and 2 days of gestation, as she presented with spontaneous rupture of membranes. The patient progressed naturally into labor and received epidural analgesia for pain relief, which was administered without complications. In a seated position, 2 mL of 2% lidocaine was used for local infiltration. After identifying the L4/5 interspace, a 16-gauge Tuohy needle was inserted, and loss of resistance to saline was confirmed at 4 cm. The epidural catheter was advanced 5 cm into the epidural space and secured at a depth of 9 cm at the skin. An initial bolus of 15 mL of 0.2% Naropin (ropivacaine) was administered in increments, followed by a continuous infusion of 0.2% Naropin at 8 mL per hour using programmed intermittent epidural bolus (PIEB) mode. The procedure was completed smoothly and uneventfully. Oxytocin infusion was used to augment labor. Due to the failure of descent of the fetal head and a prolonged second stage of labor, she underwent an emergency C-section under epidural anesthesia. The epidural anesthesia was maintained throughout the procedure without any complications. Her postoperative recovery was uncomplicated, and she was discharged on the fourth postoperative day in good condition.
On the night following her discharge, the patient presented to the emergency department with complaints of a severe, intolerable headache. She described it as a gradual onset of a throbbing, frontal headache, accompanied by intense neck pain and nausea. On examination, she appeared visibly distressed and was crying from the pain, but her Glasgow coma scale (GCS) score was 15/15. Her pupils were equal and reactive to light. Neck stiffness was noted, indicating meningeal irritation. Her blood pressure was elevated at 190/120 mm Hg, but no focal neurological deficits were observed. Given the sudden onset of a severe headache and neck stiffness, SAH was clinically suspected.
Diagnosis
A computed tomography (CT) scan of the brain revealed acute SAH, as seen by hyper-densities within the anterior interhemispheric fissure and left sylvian fissure consistent with widespread acute SAH, which typically presents as high-density areas on a non-contrast CT scan due to the presence of fresh blood within the subarachnoid space (Fig. 1). Mild diffuse edema was also noted in the left cerebral hemisphere suggesting a localized inflammatory response and possible increase in intracranial pressure (ICP). The patient was admitted to the intensive care unit for close monitoring. A subsequent computed tomography angiogram (CTA) showed no evidence of aneurysmal rupture or AVM (Fig. 2), indicating a non-aneurysmal spontaneous SAH, which is important for narrowing the differential diagnosis. Non-aneurysmal SAH may be caused by factors such as vascular fragility or hypertension, as opposed to aneurysmal rupture. Laboratory tests, including hematological, biochemical, and coagulation profiles, were all within normal limits. This further supported the diagnosis of spontaneous, non-aneurysmal SAH. This comprehensive diagnostic workup guided the management by excluding other potential causes and confirming the nature of the hemorrhage. A Doppler ultrasound of the carotid and vertebral arteries revealed no significant abnormalities.
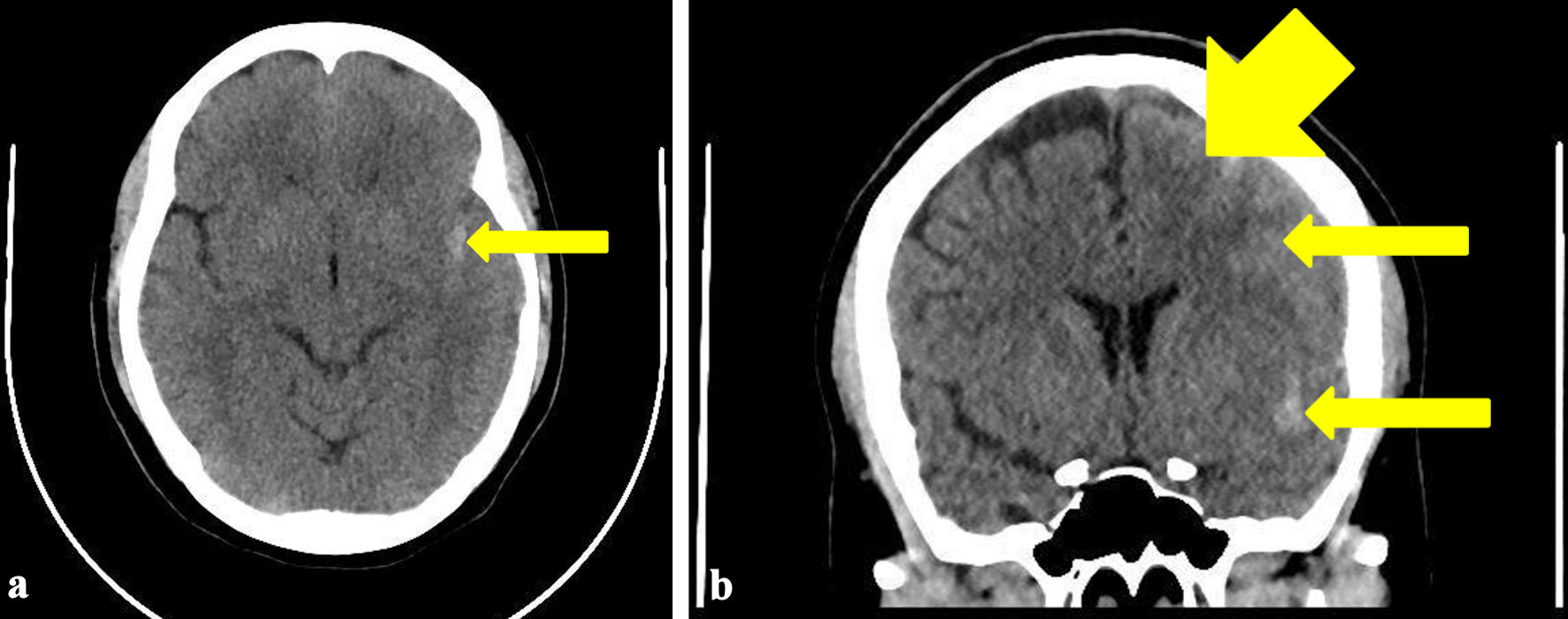 Click for large image | Figure 1. Transverse view (a) and coronal view (b) of the CT brain showing hyper-densities within left sylvian fissure (arrow) and over left anterior frontal lobe (arrows) consistent with widespread acute subarachnoid hemorrhage. Mild diffuse edema is observed in the left cerebral hemisphere. CT: computed tomography. |
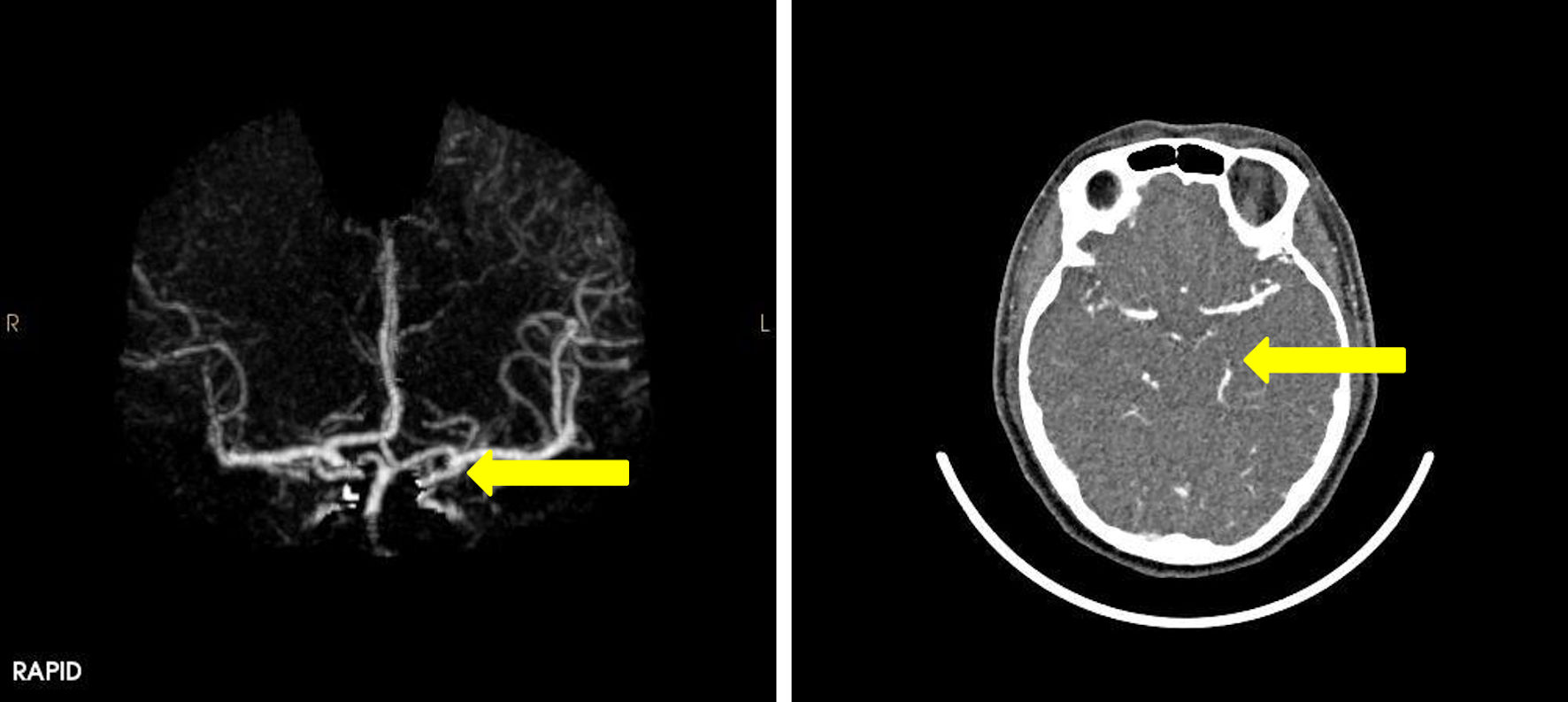 Click for large image | Figure 2. CT angiography of the brain showing normal appearance of the circle of Willis. Normal angiographic appearance of both vertebral arteries, basilar artery and posterior cerebral arteries (arrow). CT: computed tomography. |
Treatment
Supportive care was initiated, including intravenous fluids, analgesics, and nimodipine (60 mg every 4 h) to prevent cerebral vasospasm. Strict blood pressure control was maintained to reduce the risk of rebleeding. The patient was closely monitored with regular neurological assessments, including GCS evaluations. Given the absence of an aneurysm on CTA, conservative management was pursued. Over the following days, the patient’s condition improved, with a gradual reduction in headache intensity and a return to baseline neurological function. She remained stable throughout her hospital stay and was discharged on the eighth postoperative day in good health, with no residual neurological deficits.
Follow-up and outcomes
At her 6-week follow-up, the patient reported occasional mild headaches but no other symptoms. Further imaging was deemed unnecessary, as her clinical recovery was complete, and the cause of the SAH remained idiopathic.
| Discussion | ▴Top |
SAH following a C-section is an extremely rare occurrence, particularly in healthy postpartum women with no previous neurological or antenatal complications. While SAH is most commonly associated with the rupture of cerebral aneurysms, it may also occur due to AVM, trauma, or spontaneously. In the postpartum setting, the condition may be easily overlooked or misdiagnosed, especially when patients present with symptoms like headache or altered mental status, which are frequently attributed to more common postpartum issues such as preeclampsia, migraine, or even the stress of labor. The occurrence of SAH in postpartum women, though rare, presents unique challenges for clinicians in both diagnosis and management. A 2020 study by Ueda et al involving 167 patients found that the occurrence of SAH during pregnancy was less frequent in the first trimester (before 15 weeks) and significantly increased during the third trimester (30 - 40 weeks) [7]. This is due to hormonal and hemodynamic changes during pregnancy. Uterine contractions and the Valsalva maneuver, which are integral during labor, can cause temporary but considerable increases in blood pressure and ICP, including cerebrospinal fluid (CSF) pressure. These physiological shifts are driven by the rise in intra-abdominal pressure from contractions and pushing, which subsequently elevates venous pressure and decreases venous return to the heart. These abrupt blood pressure surges place significant stress on the vascular system, particularly in individuals with fragile or weakened cerebral blood vessels, heightening the risk of rupture [8]. Physiological changes during labor and cesarean delivery might increase the risk of SAH, but this link requires further research. Aneurysm rupture can be triggered by labor-induced elevations in ICP due to straining, especially in people who already have vascular abnormalities such as AVMs or aneurysms. Pregnancy-related hormonal changes, such as increased estrogen, may weaken vascular walls, increasing fragility. The postpartum hypercoagulable state, while protective against hemorrhage, might exacerbate venous congestion or unmask latent vascular abnormalities. Additionally, neuraxial anesthesia, though rare, may contribute through changes in CSF dynamics or hemodynamic fluctuations, potentially triggering vessel rupture in vulnerable patients. Clarifying these mechanisms strengthens the rationale for postpartum SAH, highlighting the need for clinical vigilance during and after delivery to effectively manage potential neurological complications. For patients with undiagnosed cerebrovascular anomalies, such as aneurysms or AVM, these repeated pressure spikes during labor may trigger SAH. Additionally, increased CSF pressure during the Valsalva maneuver may further exacerbate the strain on vulnerable cerebral vessels [9]. Although rare, this physiological response suggests a potential connection between the demands of labor and the occurrence of SAH, even in the absence of common risk factors. This emphasizes the importance of careful monitoring for neurological symptoms in postpartum women, as labor-induced pressure fluctuations combined with latent vascular abnormalities can lead to serious complications such as SAH.
In 2001, Eggert et al reported a case involving an obstetric patient who presented to the hospital for the removal of retained placenta. Following the administration of spinal anesthesia, the patient experienced a sudden and intense headache, which was later diagnosed as SAH [10]. This case illustrates the potential, albeit rare, complication of SAH following spinal anesthesia in obstetric patients. The occurrence of severe headaches after spinal anesthesia is often attributed to post-dural puncture headaches (PDPHs). However, when the headache is unusually severe or associated with other neurological symptoms, it is crucial to consider more serious underlying causes, such as SAH.
In our case, the patient had received epidural analgesia for painless labor. The procedure was completed smoothly and uneventfully. She had a prolonged second stage of labor of a duration of more than 3 h. She was taken up for an emergency C-section due to failure of descent of the fetal head and a prolonged second stage of labor. Her postoperative period during hospital stay was uneventful and she was discharged in satisfactory condition. She presented to the emergency department the same night with a throbbing headache and mild neck stiffness. After ruling out common causes of headache, a CT scan of the brain was performed, revealing an acute SAH. Despite the fact that the patient did not describe having a headache at the time, it is plausible that the SAH happened at some point during labor. This development might have been influenced by cardiovascular alterations that occur during labor. Given that many spontaneous SAHs have no apparent etiology, it is also possible that the simultaneous occurrence of labor, epidural anesthesia, and SAH was coincidental. In a case series published by Edlow et al in 2000, 34% of SAHs occurred during non-strenuous exercise, whereas 12% occurred while the patients were sleeping. However, given how uncommon SAHs are overall, such a coincidence is incredibly unlikely [11]. Intracerebral hemorrhage after lumbar puncture has not been well documented in research, especially in pregnant individuals. A lesser number of these instances reported intraparenchymal hemorrhage, while the majority had subdural hematomas [12]. There are very few case reports that describe SAH in pregnant patients following spinal anesthesia with bupivacaine.
SAH following epidural analgesia during labor is an exceedingly rare but serious complication. While epidural analgesia is generally considered safe and effective for pain relief in labor, certain complications, including SAH, can occur, typically presenting with symptoms such as sudden severe headache, nausea, vomiting, altered consciousness, or neurological deficits. The pathophysiology of SAH in this context might involve accidental dural puncture leading to bleeding, vascular abnormalities such as aneurysms, or an increase in ICP during labor. Immediate diagnosis through imaging, such as CT scan or magnetic resonance imaging (MRI) scan, is critical for management. A rare case report of a patient who experienced SAH and pneumocephaly - a dangerous but infrequent consequence of puncturing the dura mater during epidural anesthesia - was published in 2014 by Guzel et al [13]. According to a theory put forth by Bottiger et al [14], a CSF leak could result in lower ICP. Increased transmural pressure across the artery as a result may cause a rupture that results in bleeding [14]. This notion is supported by the fact that spinal and epidural anesthesia have not been recommended for patients who have intracranial vascular abnormalities or persistently low CSF systemic pressure.
In a case report published in 2020 by Yadav et al [1], a patient suffered a spontaneous SAH, 30 min after surgery. She complained of several episodes of vomiting and a strong, throbbing headache. Her symptoms continued even after receiving conservative treatment. Notably, her spinal anesthesia was administered without any complications, and she did not encounter any issues during the procedure [1]. Latchaw et al report that in three out of 10 SAHs detected on CT scans, angiography showed no discernible lesion. This often indicates a better prognosis, with a 4% chance of rebleeding [15]. Another interesting case report by Eggert et al [10] reported a patient that had spinal anesthesia and was later diagnosed with SAH. The angiography in their case was normal, and he reported that the occurrence of SAH with spinal anesthesia was quite incidental [10]. He reported that vasopressors, such as ephedrine, may also raise mean arterial pressure (MAP), which could subsequently cause the aneurysm to burst [10]. However, in our case, ephedrine was not given.
The risk of SAH increases with multiple attempts at epidural or spinal anesthesia due to the potential trauma caused by repeated needle insertions. Each attempt can lead to damage or rupture of blood vessels near the spinal cord, especially small, delicate vessels within the spinal canal. Additionally, these repeated punctures may disturb the pressure balance between the CSF and the surrounding blood vessels. Rapid pressure changes can stress the vessel walls, increasing the likelihood of rupture and resulting in bleeding into the subarachnoid space. This combination of mechanical trauma and pressure instability raises the risk of SAH [16]. In our patient, epidural analgesia was achieved in the first attempt.
In the postpartum period, several conditions can mimic the presentation of SAH, necessitating careful differential diagnosis. These include PDPH, a common cause of postpartum headache, particularly following epidural or spinal anesthesia, which is typically characterized by positional headache and relief on lying down. In this case, the absence of positional headache and the presence of severe, thunderclap headache with neurological deficits, such as neck stiffness, raised suspicion of an alternative diagnosis.
Hypertensive disorders in the postpartum period, including eclampsia, can present with severe headaches, seizures, and altered mental status. However, this patient had normal blood pressure readings throughout the pregnancy and postpartum period, with no proteinuria or other signs of preeclampsia, making this diagnosis unlikely.
Headaches can also be a symptom of infectious conditions like encephalitis and meningitis. Other symptoms, such as fever and stiff neck, are usually linked to these disorders, however these were not present in this patient. Notably, the patient was afebrile and showed no other systemic signs of infection.
Cerebral venous sinus thrombosis (CVST) can present with postpartum headache and neurological symptoms, especially in women with hypercoagulable states. Imaging studies ruled out venous sinus thrombosis in this case.
While migraines and cluster headaches are common in the postpartum period, the acute onset, severity, and associated neurological symptoms in this case were inconsistent with these diagnoses.
The combination of a sudden, throbbing, frontal headache, accompanied by intense neck pain and nausea in the absence of fever or infection raises strong suspicion for SAH. A CT scan of the head confirmed the presence of blood in the subarachnoid space, establishing the diagnosis.
A headache and bleeding sensation are caused by the CSF leaking from the dural hole, which alters the CSF dynamics and traction of the dura, cranial nerves, and bridge veins. The following four factors can cause bleeding: AVM, anticoagulant use, dehydration, brain atrophy, and significant CSF leakage. Our patient’s coagulation profile was normal despite the SAH. Post C-section, she was on a prophylactic anticoagulant to prevent deep vein thrombosis. This also enhances the likelihood of developing a hemorrhage. During the recovery phase, she was hydrated well. Throughout administration of epidural analgesia, there is typically no direct puncture of the dura mater, which means CSF leakage is less likely compared to spinal administration. However, if the dura is accidentally punctured during the epidural procedure (a “wet tap”), CSF can leak into the epidural space, leading to potential complications like PDPH. Adequate hydration, such as with Ringer lactate solution, helps maintain overall fluid balance but does not directly prevent CSF loss if a wet tap occurs. Therefore, CSF loss is not a common issue in standard epidural analgesia use unless a dural puncture happens. Our patient had an uneventful and uncomplicated epidural administration.
Another case report by D’Angelo et al (2014) aligns with documented cases of postpartum SAH associated with risk factors such as hypertensive disorders, structural anomalies (e.g., aneurysms or AVMs), or significant vascular changes during pregnancy, but adds uniqueness due to its occurrence following epidural anesthesia without preceding symptoms [17].
This case report underscores the rarity of SAH following a C-section, particularly in patients with no prior neurological symptoms or risk factors. While SAH is uncommon in the postpartum period, the physiological changes during pregnancy and after delivery may increase the risk of vascular events. This case serves as a reminder of the complexities that can arise in obstetric care and the importance of integrating neurological evaluation when symptoms suggest a potential vascular event.
Learning points
This case highlights the rare occurrence of spontaneous SAH in the immediate postpartum period following an emergency C-section. While SAH is typically associated with aneurysmal rupture, this case of non-aneurysmal spontaneous SAH underscores the importance of considering this diagnosis in postpartum patients presenting with sudden, severe headaches, even in the absence of prior neurological symptoms or significant medical complications during pregnancy. Early recognition, prompt neuroimaging, and appropriate management, including supportive care and blood pressure control, can lead to favorable outcomes. Further research is necessary to understand the potential postpartum physiological factors that may contribute to increased vulnerability to cerebrovascular events like SAH.
Acknowledgments
We thank Mediclinic Middle East for granting permission to publish this case.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Written informed consent was obtained from the patient for publication of this case report.
Author Contributions
Conceptualization: Jijisha Ali, Dora Dimarucut, Rida Maryum. Investigation: Jijisha Ali, Dora Dimarucut, Shriganesh Shankarrao Patil. Formal analysis: Jijisha Ali. Supervision: Jijisha Ali, Dora Dimarucut. Writing - original draft: Jijisha Ali. Writing - review and editing: Jijisha Ali, Dora Dimarucut, Rida Maryum, Shriganesh Shankarrao Patil, Tejal Desai.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
SAH: subarachnoid hemorrhage; C-section: cesarean section; AVM: arteriovenous malformation; GCS: Glasgow coma scale; CT: computed tomography; MRI: magnetic resonance imaging; CTA: computed tomography angiography; CSF: cerebrospinal fluid; MAP: mean arterial pressure
| References | ▴Top |
- Yadav A, Agrawal A, Sharma R. Spontaneous subarachnoid haemorrhage in an obstetric patient post spinal anaesthesia. Ann Indian Acad Neurol. 2020;23(6):838-840.
doi pubmed - Petridis AK, Kamp MA, Cornelius JF, Beez T, Beseoglu K, Turowski B, Steiger HJ. Aneurysmal Subarachnoid Hemorrhage. Dtsch Arztebl Int. 2017;114(13):226-236.
doi pubmed - Duong H, Melancon D, Tampieri D, Ethier R. The negative angiogram in subarachnoid haemorrhage. Neuroradiology. 1996;38(1):15-19.
doi pubmed - Mas JL, Lamy C. Stroke in pregnancy and the puerperium. J Neurol. 1998;245(6-7):305-313.
doi pubmed - Gorelick PB, Hier DB, Caplan LR, Langenberg P. Headache in acute cerebrovascular disease. Neurology. 1986;36(11):1445-1450.
doi pubmed - Saad Eddin A, Shaikh N. Aneurysmal subarachnoid hemorrhage after cesarean section under spinal anesthesia: a case report. Research Square. 2022.
doi - Ueda T, Kiura Y, Isobe N, Nishimoto T. A patient with subarachnoid hemorrhage related to a ruptured aneurysm in week 8 of pregnancy: usefulness of coil embolization of intracranial aneurysms as a treatment option before delivery. J Neuroendovasc Ther. 2020;14(1):30-35.
doi pubmed - Horton JC, Chambers WA, Lyons SL, Adams RD, Kjellberg RN. Pregnancy and the risk of hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1990;27(6):867-871; discussion 871-862.
doi pubmed - Hunt HB, Schifrin BS, Suzuki K. Ruptured berry aneurysms and pregnancy. Obstet Gynecol. 1974;43(6):827-837.
pubmed - Eggert SM, Eggers KA. Subarachnoid haemorrhage following spinal anaesthesia in an obstetric patient. Br J Anaesth. 2001;86(3):442-444.
doi pubmed - Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med. 2000;342(1):29-36.
doi pubmed - Newrick P, Read D. Subdural haematoma as a complication of spinal anaesthetic. Br Med J (Clin Res Ed). 1982;285(6338):341-342.
doi pubmed - Guzel M, Salt O, Erenler AK, Baydin A, Demir MT, Yalcin A, Doganay Z. Subarachnoid hemorrhage and pneumocephalus due to epidural anesthesia. Am J Emerg Med. 2014;32(8):945.e945-947.
doi pubmed - Bottiger BW, Diezel G. [Acute intracranial subarachnoid hemorrhage following repeated spinal anesthesia]. Anaesthesist. 1992;41(3):152-157.
pubmed - Latchaw RE, Silva P, Falcone SF. The role of CT following aneurysmal rupture. Neuroimaging Clin N Am. 1997;7(4):693-708.
pubmed - Shay A, Liu L, Chu A, et al. Subarachnoid hemorrhage following spinal anesthesia for cesarean sections. MOJ Womens Health. 2016;2(4):89-91.
doi - D'Angelo R, Smiley RM, Riley ET, Segal S. Serious complications related to obstetric anesthesia: the serious complication repository project of the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2014;120(6):1505-1512.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.