| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://jcgo.elmerpub.com |
Case Report
Volume 15, Number 1, March 2026, pages 41-47
Rethinking Treatment Options in Lumbosacral Endometriosis: Positive Outcomes With Hormonal Therapy in Four Cases
Carlotta Isabella Zitzmanna, Sylvia Mechsnera, b
aDepartment of Gynecology Charite with Centre of Oncological Surgery, Endometriosis Research Centre; Charite, Campus Virchow-Klinikum, 13353 Berlin, Germany
bCorresponding Author: Sylvia Mechsner, Department of Gynecology Charite with Center of Oncological Surgery, Endometriosis Research Center; Charite, Campus Virchow-Klinikum, 3353 Berlin, Germany
Manuscript submitted December 1, 2025, accepted January 16, 2026, published online February 7, 2026
Short title: Hormones for Lumbosacral Endometriosis
doi: https://doi.org/10.14740/jcgo1607
| Abstract | ▴Top |
Neural endometriosis can be a rare form of deep infiltrating endometriosis (DIE) involving the plexus lumbosacralis, often causing neuropathic pain and pelvic organ dysfunction. Traditionally, surgical excision has been the primary treatment approach; however, hormonal therapy is gaining preference due to its lower morbidity. This study aims to assess the effectiveness of hormonal therapy for this condition. This retrospective case series included four women treated at the Endometriosis Center Charite, all of whom had imaging- or histologically confirmed lumbosacral endometriosis. The patients received either hormonal therapy alone or a combination of hormonal therapy and surgery. Symptom progression was evaluated from clinical records and classified using a four-point symptom severity scale for pain, organ dysfunction, and mobility. Outcomes were illustrated using heat maps to compare symptom severity before and after treatment. Four patients with imaging- or histologically confirmed lumbosacral endometriosis were included in this case series. All four patients received hormonal therapy. Two of the patients were treated exclusively with uninterrupted hormonal treatment and did not undergo surgery; both experienced significant symptom relief and achieved full functional recovery. The other two patients required surgical intervention due to severe disease manifestations, which included motor deficits or organ obstruction. Although surgery resulted in pain reduction, their neurological recovery was incomplete, and they continued to experience persistent motor or sensory deficits. In both cases, ongoing postoperative hormonal therapy led to further improvement in their symptoms. The most favorable outcomes were observed in the two patients who received uninterrupted hormonal treatment without surgery, both of whom achieved full functional recovery. In this case series, hormonal therapy effectively alleviated symptoms of lumbosacral endometriosis, often with fewer complications than surgery. Surgical intervention may be required when nerve compression causes motor impairment or if hormonal therapy fails. Early and ongoing hormonal treatment seems to be a safe and effective approach for reducing pain and organ-related dysfunction while maintaining quality of life.
Keywords: Lumbosacral endometriosis; Sacral endometriosis; Deep infiltrating endometriosis; Hormonal therapy; Medical management; Non-surgical treatment; Pain reduction; Chronic pain
| Introduction | ▴Top |
Endometriosis affects around 10% of women of reproductive age and is defined by the presence of endometrial-like tissue outside the uterus [1]. In about 90% of patients, the disease remains confined to the pelvic peritoneum or the ovaries. In a smaller part of patients, deep infiltrating lesions can grow into adjacent organs like bowel, vaginal, bladder, or ureter; however, in a tiny minority, lesions can also infiltrate the lumbosacral plexus or its peripheral branches. Sacral nerve root endometriosis often occurs as part of parametric deep infiltrating endometriosis (DIE) and usually affects the S2 to S4 nerves [2]. These nerves contribute to the innervation of the bladder, rectum, genital organ, and the sigmoid and descending colon [3]. This explains why most of the patients diagnosed with lumbosacral endometriosis presented with endometriosis-related pain and cyclic digestive complaints, including dysmenorrhea, deep dyspareunia, constipation, and dyschezia [2]. Involvement of the inferior hypogastric plexus can provoke bladder, rectal, and left-sided colonic dysfunction and vaginal dryness. At the same time, infiltration of the S2–S4 roots explains cyclic neuropathic pain and pain perception along the course of the sciatic nerve [4]. Whereas visceral pain is typically vague and poorly localized, sacral radiculopathy produces a sharply defined, burning or stabbing dysesthesia, often accompanied by paresthesia and hyperesthesia [5]. Persistent irritation may result in protective antalgic postures with secondary motor weakness and risk of the pain becoming chronic [6]. Although extremely rare, lumbosacral endometriosis is clinically significant because its invasion of neural and visceral structures can cause neuropathic pain and dysfunction in related organs.
If the neuropathic pain caused by neural endometriosis is left untreated, it will lose its cyclical nature and become persistent and unmanageable by analgesics [7]. Hormonal therapy is the first-line treatment for endometriosis, as both progesterone and GnRH agonists/antagonists have proven to be effective, safe, and suitable for long-term use, while generally being well tolerated [8, 9]. Nonetheless, in roughly one-third of women, hormonal treatment is ineffective or not tolerated due to side effects such as irregular bleeding, weight gain, reduced libido, or headache [10]. Although the hormones work well on the endometrial lesions and the smooth muscles of the nodules, they do not manage to treat the fibrotic tissue surrounding the nerves [11]. When pain and functional impairment persist despite medical therapy and the patient’s quality of life remains compromised, operative management becomes necessary [2, 5]. So laparoscopic excision is considered the best approach for both diagnosing and treating DIE of the sacral nerve roots [2]. Resection of deep-infiltrating lesions may reduce pain and neurological symptoms, as well as bladder- and bowel-related symptoms [5]. However, such radical surgery is technically demanding. It carries appreciable intra- and postoperative morbidity, including damage to the autonomic innervation (hypogastric plexus) with ensuing autonomic dysfunction, and should be undertaken only in specialized multidisciplinary centers [2, 5].
Furthermore, damage to the somatic nerves will risk paralysis and motoric dysfunction, and scar tissue can provoke extensive postoperative neuropathic pain [4, 7]. In addition, endometriosis is a chronic, potentially progressive disease, and repeated operations increase the risk of adhesions, fibrosis, and chronic pain, with recurrence rates after surgery reaching 34.7% within 5 years [12, 13]. Therefore, in this retrospective case analysis, we aimed to investigate the effectiveness of hormonal therapy in alleviating pain and organ-related symptoms in patients with lumbosacral endometriosis. Motor dysfunction and other functional outcomes were also evaluated and compared with those observed following surgical therapy. Specifically, this study addresses the question of how symptoms improve in patients treated with hormonal therapy alone, in comparison to surgical intervention or a combined therapeutic approach.
| Case Reports | ▴Top |
Study design and setting
To evaluate and classify patient outcomes, a retrospective qualitative case analysis was conducted on women diagnosed with lumbosacral endometriosis and treated at the certified Endometriosis Centre at Charite Universitatsmedizin Berlin. Eligible cases were identified through electronic medical records and included women aged 20 to 40 years at first diagnosis, with lumbosacral endometriosis confirmed by imaging or histology, who had received medical (hormonal) therapy and had documented follow-up of at least 18-month to 6 years with symptom reporting. Patients were excluded if follow-up data were incomplete or if hormonal treatment had not been administered. Using the Charite hospital information system, all relevant documents and imaging findings were reviewed and incorporated into the evaluation of the patients. Particular attention was given to the trajectory of pain, including narrative descriptions of pain intensity, numerical ratings on a 0–10 visual analogue scale when available, and changes in analgesic consumption. Organ-related dysfunction was also carefully assessed, encompassing bladder and bowel disturbances, sexual dysfunction, neurogenic symptoms such as paresthesia or hyperesthesia in the lower limbs, and limitations in mobility. Due to the small cohort size and incomplete quantitative pain scores, formal statistical analyses were not performed. Instead, outcomes are presented as individual qualitative narratives, supplemented with graphical representations to highlight patterns in pain relief, organ function, and mobility before and after treatment. All narrative chart entries on pain, bladder and bowel dysfunction, and mobility were converted into an ordinal four-point scale (0 = none, 1 = mild, 2 = moderate, 3 = severe), which was then used to generate heat maps illustrating symptom severity prior to and following hormonal therapy. All patients provided informed consent for the presentation of their individual medical histories.
Case 1
This female patient (G1/P1) received a first histological diagnosis of sacral endometriosis at 34 years old in February 2023 during a diagnostic laparoscopy that documented a right-sided pararectal nodule infiltrating the sacral plexus and sciatic nerve (Fig. 1). The laparoscopic procedure involved biopsy of the pararectal lesion but no radical nerve dissection. Pelvic magnetic resonance imaging (MRI) in February 2023 demonstrated a 4 × 3 cm spiculated, deeply infiltrating endometriotic mass extending from the upper vaginal wall with contact to the S3 nerve root. Her symptoms included right gluteal pain, a voiding disorder, dysmenorrhea accompanied by nausea and vomiting, and paresthesia of the right leg and foot. She also had complaints of bowel dysfunction with cyclical diarrhea, flatulence, and dyschezia. After diagnosis, continuous treatment with dienogest 2 mg once a day was started immediately and increased in April 2023 to twice a day due to persistent vaginal bleeding. Four months after the baseline scan, the repeat MRI showed a regression of lesion volume, although the mass still tracked along retroperitoneal vessels and touched the S3 nerve and the sciatic nerve. Faced with the option of a radical nerve-sparing excision, the patient declined further surgery in June 2023 after counselling about the high risk of sensory and autonomic deficits. Instead, she continued medical therapy, but, although her symptoms had improved, she switched to GnRH-analogues (leuprorelin) and dienogest 2 mg twice a day due to persistent bleeding and an estradiol level of 40.8 pg/mL. This value, although formally within the proposed therapeutic estradiol window during dienogest treatment, was considered relatively high within the target range of ∼30–50 pg/mL, which has been described as sufficient to suppress endometriotic lesion activity while avoiding profound hypoestrogenic side effects associated with deeper estrogen suppression [14]. In the first few weeks, her pain stabilized and the estradiol level dropped to 13.2 pg/mL, but a slight paresthesia persisted.
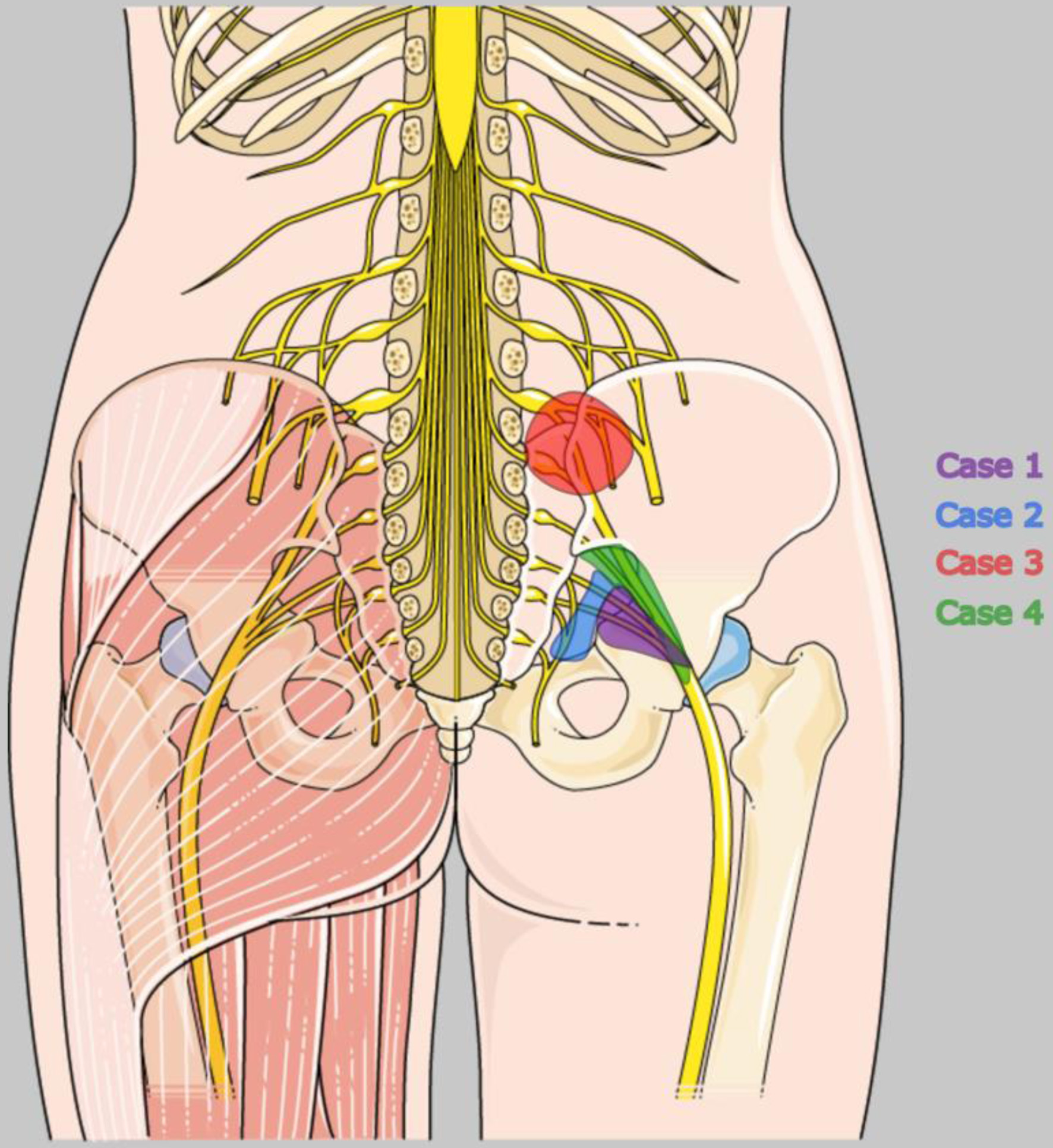 Click for large image | Figure 1. Localization of endometriotic lesions affecting the lumbosacral plexus in the four patients presented in this case report. Illustration adapted from Servier Medical Art (https://smart.servier.com/), licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). |
The patient wished to have another child and visited a fertility center in July 2023. However, due to a low anti-Mullerian hormone (AMH) level, she was informed that her ovarian reserve was insufficient, making conception unlikely. In December 2023, her medical therapy was switched to a gonadotropin-releasing hormone (GnRH)-receptor antagonist combination (relugolix combined therapy ).
Nearly a year later, in May 2024, the patient reported experiencing only rare spotting and felt “almost symptom-free”. She noted that her lower limb paresthesia was barely noticeable. However, her hormonal therapy was changed back to leuprorelin and dienogest in May 2024 because her estradiol levels were measured at 51.7 pg/mL, which was outside the therapeutic window. After 5 months, her estradiol levels dropped to < 5 pg/mL, leading to the implementation of transdermal estrogen replacement. By March 2025, her symptoms remained stable, and she reported being symptom-free. In 2025, she also began to accept that her family planning was complete and that it was unlikely she would have a second pregnancy.
Case 2
The patient, a 27-year-old (G0), underwent a laparoscopic appendectomy in 2012 at another hospital, during which pelvic endometriosis was unexpectedly discovered. A second laparoscopy was performed at a different hospital in February 2021 with the aim of achieving radical excision of extensive DIE, including bilateral hypogastric nerve neurolysis. Despite this extensive surgery, she continued to experience right-sided sciatic pain radiating down her leg, stress-dependent dysmenorrhea (with pain levels ranging from 2 to 8 on a scale of 10), dyspareunia, and difficulty voiding when her pain peaked. She also faced severe limitations on sitting, cycling, and walking due to chronic neuropathic pain in her right leg, requiring regular use of non-steroidal anti-inflammatory drugs (NSAIDs) and occasionally becoming bedridden.
Imaging conducted in September 2023 (computed tomography) and October 2024 (endosonography) revealed an infiltrative nodule in the right rectovaginal septum, infiltrating the rectum, along with multifocal parametrial and adnexal lesions consistent with suspected right-sided sacral plexus involvement (Fig. 1). Due to her previous intolerance to progestin-only or combined oral contraceptives, she declined medical treatment in 2021. However, as her symptoms worsened, she agreed to start a GnRH-receptor antagonist combination (relugolix, once a day) in July 2024. Within 1 month, she became amenorrheic, discontinued all analgesics, and reported a significant decrease in leg and pelvic pain. By November 2024, she continued the medical therapy unchanged and described herself as being “pain-controlled” with “few side effects”.
Case 3
This 32-year-old patient (G0) first underwent laparoscopic surgery for left ovarian cyst enucleation and adhesiolysis involving the sigmoid colon (“sigma”) in May 2020. Afterward, she experienced progressive, cyclical neuropathic pain radiating along the femoral and genitofemoral nerves on the right side. This pain was accompanied by paresthesia, intermittent leg weakness, and significant restrictions in her ability to walk. The pain affected her right hip and radiated with a burning sensation into the thighs, extending to the knees and the lumbar spine. Additionally, she suffered from dysmenorrhea, which was accompanied by nausea starting on the fourth or fifth day of her cycle and lasting for up to 14 days.
A pelvic MRI in May 2021 revealed a muscle-invasive extragenital endometriotic mass within the right iliopsoas muscle that wrapped around the extraforaminal L4 nerve root (Fig. 1). There was also a grade 3–4 hydronephrosis on the left side, indicating ureteral stenosis caused by additional deep lesions. Neurological examination documented flaccid paresis of the quadriceps, an absent patellar reflex, and sensory loss along the L4 dermatome. These findings confirmed the presence of lumbosacral plexus endometriosis.
The patient had been using combined oral contraceptives for years, but she discontinued them to pursue motherhood, which coincided with the worsening of her symptoms. In June 2021, she began treatment with dienogest 2 mg once daily, and within weeks, her leg pain improved significantly. In her desire to conceive, the patient underwent five frozen embryo transfers in 2022, all of which were unsuccessful. Due to repeated interruptions in her dienogest treatment for fertility treatments, she experienced a gradual return of her neuropathic symptoms throughout 2022. After the failure of all assisted reproductive treatments (ARTs), she decided to stop ART and resume hormonal therapy with dienogest.
By August 2023, she reported being “able to live quite normally,” needing analgesics only twice a month. However, due to persistent left-sided ureteral stenosis and hydronephrosis, the patient opted for surgery. In December 2023, she underwent laparoscopy, which included extensive adhesiolysis, left ureter resection with end-to-end anastomosis over an Allium stent, segmental bowel shaving, and left adnexectomy; no dedicated nerve dissection was attempted.
After her unsuccessful attempts at ART, she chose to adopt and welcomed a baby in August 2024. By July 2025, the patient was clinically stable on continuous hormonal therapy with dienogest, allowing her to manage daily activities without the need for pain medication. However, she still experienced occasional mild symptoms. She also noted that switching between manufacturers (same active ingredient, dienogest) could temporarily worsen her nerve-related pain.
Case 4
A 27-year-old woman (G1/P1) was diagnosed in 2016 with DIE, which formed a cystic mass measuring 4.5 × 4 × 3.5 cm that encased the right sciatic nerve at the greater sciatic foramen. Imaging also revealed atrophy of the piriformis and obturator internus muscles (Fig. 1). Her primary complaints included dysmenorrhea and persistent, previously cyclical sciatic pain radiating from the right buttock down her leg. She exhibited an antalgic posture with functional leg-shortening and progressive foot drop.
In March 2016, she began treatment with continuous dienogest at a dosage of 2 mg twice a day. An MRI conducted in March 2017 showed only a minimal reduction in the cyst’s size, while the nerve remained fully encased. By June 2017, a neurological examination indicated hip-joint restriction and a partial lesion of the peroneal branch of the sciatic nerve, resulting in 4/5 weakness of the dorsiflexors and associated muscle atrophy. By this point, surgeons recommended prompt intervention to preserve nerve function.
Although the patient had been stable on dienogest, she discontinued the hormonal therapy due to her desire to conceive. After stopping the medication, her symptoms worsened significantly; she was limited to short walks due to neuropathic pain, experienced spotting, and relied on daily analgesics. These issues led to laparoscopic neurolysis with partial sciatic nerve resection and pelvic wall peritonectomy in December 2017. During the surgery, a chocolate-fluid cyst conglomerate was dissected away from the nerve as it extended through the greater sciatic foramen.
Postoperatively, the patient was pain-free, could elevate her right leg unaided, and began physiotherapy-assisted walking exercises. However, she experienced a transient worsening of foot drop and sensory loss. To prevent recurrence, dienogest at 2 mg twice a day was restarted immediately after surgery. Three months later, her pain had completely resolved, and she was able to lift her leg; however, the foot drop and sensory loss in her distal leg persisted.
After surgery, the patient developed a decubitus ulcer on her right heel (Fig. 2a), likely due to neuropathic and trophic disturbances resulting from sciatic nerve injury. The associated loss of protective sensation and motor function led to prolonged unperceived pressure on the affected area, delaying healing, which took approximately 1 year (Fig. 2b).
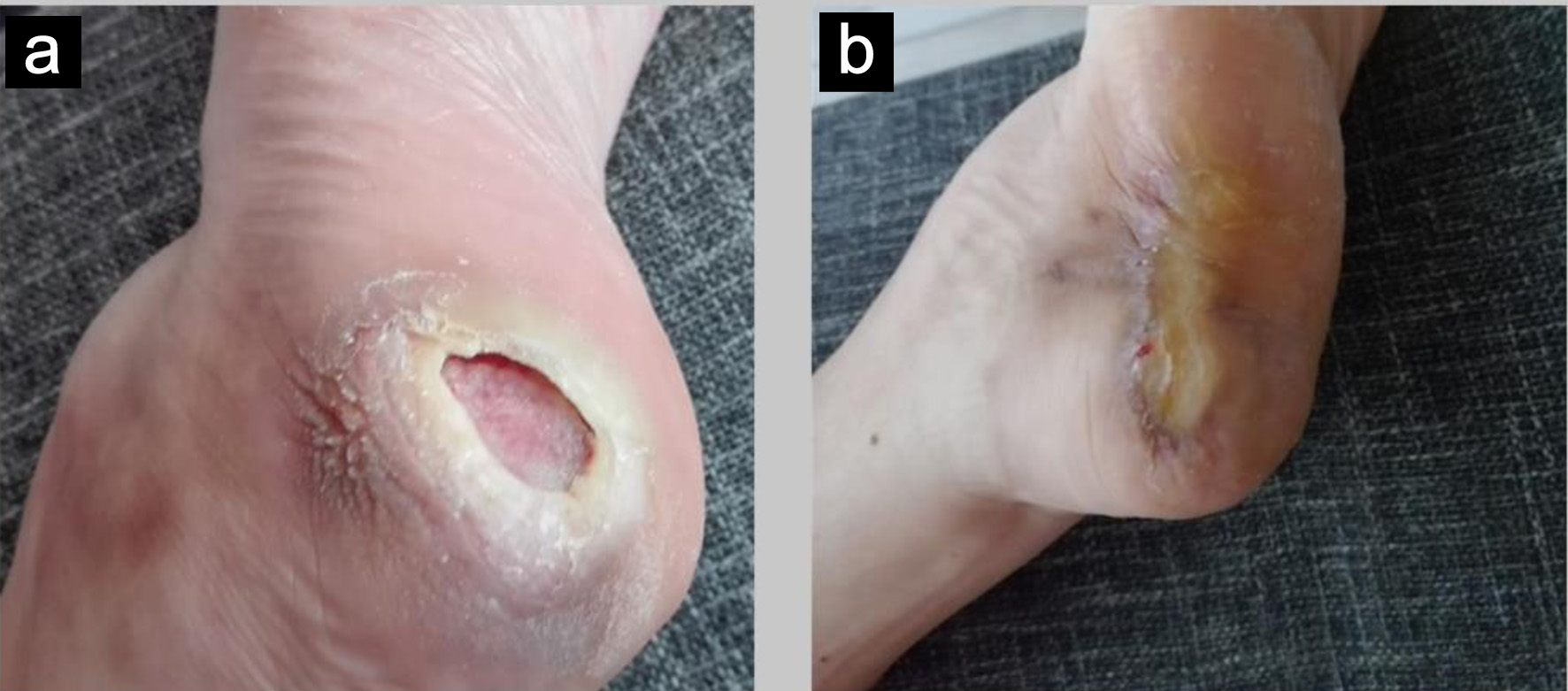 Click for large image | Figure 2. Clinical presentation of the right heel pressure ulcer in 2018 (a) and its progression 1 year later in 2019 (b). |
By May 2018, the patient could walk short distances unaided, using crutches only for longer distances, although paresthesia in her right foot had not improved. A neurological examination confirmed a proximal sciatic nerve lesion with signs of denervation. An MRI in July 2018 showed no recurrent endometriosis; however, a fibrotic scar measuring 15 × 20 mm encased the sciatic nerve, which remained intact morphologically. Her pelvic muscles were still atrophic, indicating a slow neurological recovery.
Due to occasional spotting indicating incomplete ovarian suppression and ongoing complaints, the dosage of dienogest was doubled to 4 mg twice a day in November 2019. Since the patient still desired to have children, she underwent ART, resulting in the birth of a healthy baby in 2023. As of July 2025, the patient continues her hormonal therapy with dienogest twice daily, and her sciatic pain has resolved. However, she has developed equinus foot syndrome and requires a foot orthosis for walking. Additionally, she continues to experience mild sensory disturbances in her right leg.
| Discussion | ▴Top |
In neural endometriosis, first-line treatment is often surgical due to limited data on hormonal therapy as an alternative. However, extensive neural infiltration carries high surgical risk, including irreversible sensory and motor deficits [4, 5, 7]. In this series of four women, continuous hormonal therapy consistently improved symptoms, as shown in the heat map (Fig. 3). Maintaining estrogen levels within 30–50 pg/mL was critical; suboptimal suppression resulted in persistent bleeding and pain.
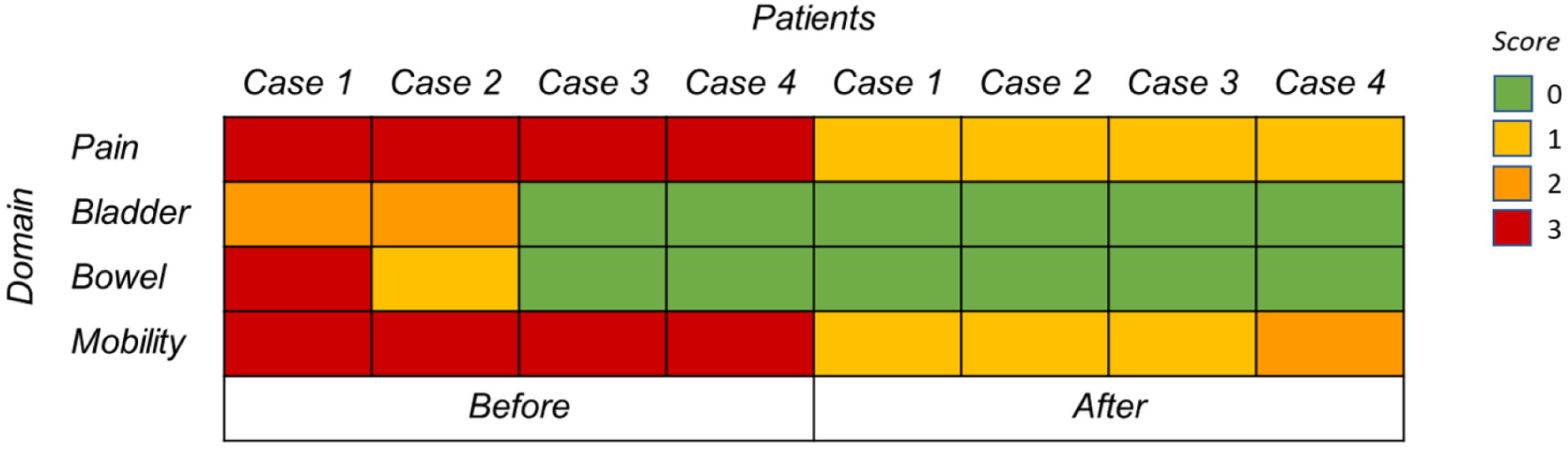 Click for large image | Figure 3. Heat map illustrating symptom severity in four women with sacral endometriosis before (left) and after (right) treatment. Severity levels: 0 = none, 1 = mild, 2 = moderate, 3 = severe. |
The heat map revealed a shift from severe (red) to mild or absent (yellow-green) symptoms after therapy, demonstrating improvements in pain, bowel, bladder, and motor function. These cases suggest early, uninterrupted hormonal therapy can effectively control lumbosacral plexus endometriosis in most patients. Second-line therapies such as GnRH-antagonists or -analogues may be required if estrogen suppression with dienogest is insufficient.
Meta-analyses confirm dienogest reduces dysmenorrhea, non-menstrual pelvic pain, and deep dyspareunia while shrinking lesions in DIE [15, 16]. In our cohort, case 3 mirrored these benefits, achieving rapid pain relief and decreased analgesic use. However, hormones do not address perineural fibrosis [11]. When fibrosis compresses the lumbosacral plexus, minimally invasive excision can relieve pain and restore organ-related and motor functions [5]. Surgical series report significant reductions in visual analogue scale (VAS) pain scores [17, 18], but procedures are technically demanding with risks of bleeding, autonomic nerve injury, and persistent sensory or motor deficits [4, 5, 7].
Endometriosis recurrence and repeated surgery increase adhesions, fibrosis, and risks to ovarian reserve [12, 19]. Radical nerve-sparing resection should be reserved for refractory cases in specialized centers [4]. Evidence supports combining surgery with hormonal therapy to maximize the durability of relief and prevent recurrence [20], as seen in case 4.
Overall, both hormonal therapy and surgical excision can alleviate pain in lumbosacral endometriosis. Surgery may offer more profound and longer-lasting relief but carries a higher complication risk [10, 12]. A stepwise approach, initiating medical therapy and reserving surgery for refractory cases, balances efficacy with safety. Notably, lumbosacral endometriosis can occur independently of deep infiltrating disease, and evidence for hormonal therapy in these cases remains scarce, highlighting the relevance of this series [21].
The study has several limitations, including a small sample size, a retrospective design, incomplete clinical records, varied treatment regimens, and inconsistent follow-up. Additionally, the outcomes were assessed subjectively, which limits the generalizability of the findings. Despite these limitations, the series shows that continuous and adequate hormonal therapy can significantly reduce pain and improve both organ-related and motor function while minimizing surgical risks. However, surgery is still indicated when dense perineural fibrosis hinders the effectiveness of medical treatment. To establish thresholds for surgical intervention, prospective studies with standardized protocols are needed.
Acknowledgments
The authors sincerely thank the four patients who generously agreed to share their medical journeys for this study. Their willingness to contribute their experiences made this work possible and is deeply appreciated.
Financial Disclosure
None to declare.
Conflict of Interest
The authors report no conflict of interest.
Informed Consent
Written informed consent was obtained from all patients included in the case series.
Author Contributions
Carlotta Isabella Zitzmann was responsible for investigation, data curation, formal analysis, and writing the original draft. Prof. Dr. med. Sylvia Mechsner contributed to conceptualization, supervision, clinical guidance, and review and editing of the manuscript. Both authors have read and approved the final version of the manuscript.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ART: assisted reproductive treatment; CT: computed tomography; DIE: deep infiltrating endometriosis; G/P: gravida/para; MRI: magnetic resonance imaging; NSAID: non-steroidal anti-inflammatory drug; relugolix combined therapy: ; SAP: Systems, Applications, and Products in Data Processing; VAS: visual analogue scale
| References | ▴Top |
- Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10(5):261-275.
doi pubmed - Kale A, Baydili KNS, Keles E, Gundogdu E, Usta T, Oral E. Comparison of isolated sciatic nerve and sacral nerve root endometriosis: a review of the literature. J Minim Invasive Gynecol. 2022;29(8):943-951.
doi pubmed - Sharabi AF, Carey FJ. Anatomy, Abdomen and Pelvis, Splanchnic Nerves. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2025.
pubmed - Roman H, Dehan L, Merlot B, Berby B, Forestier D, Seyer-Hansen M, Abo C, et al. Postoperative outcomes after surgery for deep endometriosis of the sacral plexus and sciatic nerve: a 52-patient consecutive series. J Minim Invasive Gynecol. 2021;28(7):1375-1383.
doi pubmed - Hudelist G, Darici Kurt E, Szabo G, Miklos D, Hudelist T, Bokor A. Surgical outcomes of women undergoing radical resection of deep endometriosis of the sacral plexus: A prospective cohort study. Acta Obstet Gynecol Scand. 2025;104(1):95-101.
doi pubmed - Gharaei H, Gholampoor N. The role of interventional pain management strategies for neuropathic pelvic pain in endometriosis. Pain Physician. 2023;26(5):E487-E495.
pubmed - Possover M. Five-year follow-up after laparoscopic large nerve resection for deep infiltrating sciatic nerve endometriosis. J Minim Invasive Gynecol. 2017;24(5):822-826.
doi pubmed - Ferrero S, Alessandri F, Racca A, Leone Roberti Maggiore U. Treatment of pain associated with deep endometriosis: alternatives and evidence. Fertil Steril. 2015;104(4):771-792.
doi pubmed - (DGGG) DGfGuG. S2k-Leitlinie Diagnostik und Therapie der Endometriose. 2025. Available from: https://register.awmf.org/de/leitlinien/detail/015-045.
- Berlanda N, Somigliana E, Frattaruolo MP, Buggio L, Dridi D, Vercellini P. Surgery versus hormonal therapy for deep endometriosis: is it a choice of the physician? Eur J Obstet Gynecol Reprod Biol. 2017;209:67-71.
doi pubmed - Vercellini P, Sergenti G, Buggio L, Frattaruolo MP, Dridi D, Berlanda N. Advances in the medical management of bowel endometriosis. Best Pract Res Clin Obstet Gynaecol. 2021;71:78-99.
doi pubmed - Rezende GP, Venturini MC, Kawagoe LN, Yela Gomes DA, Benetti-Pinto CL. Surgery vs. hormone-based treatment for pain control in deep infiltrating endometriosis: a retrospective cohort study. Curr Med Res Opin. 2022;38(4):641-647.
doi pubmed - Yela DA, Vitale SG, Vizotto MP, Benetti-Pinto CL. Risk factors for recurrence of deep infiltrating endometriosis after surgical treatment. J Obstet Gynaecol Res. 2021;47(8):2713-2719.
doi pubmed - Bizzarri N, Remorgida V, Leone Roberti Maggiore U, Scala C, Tafi E, Ghirardi V, Salvatore S, et al. Dienogest in the treatment of endometriosis. Expert Opin Pharmacother. 2014;15(13):1889-1902.
doi pubmed - Wu H, Liu JJ, Ye ST, Liu J, Li N. Efficacy and safety of dienogest in the treatment of deep infiltrating endometriosis: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2024;297:40-49.
doi pubmed - Ma Y, Wang WX, Zhao Y. Dienogest in conjunction with GnRH-a for postoperative management of endometriosis. Front Pharmacol. 2024;15:1373582.
doi pubmed - Lemos N, D'Amico N, Marques R, Kamergorodsky G, Schor E, Girao MJ. Recognition and treatment of endometriosis involving the sacral nerve roots. Int Urogynecol J. 2016;27(1):147-150.
doi pubmed - Chiantera V, Petrillo M, Abesadze E, Sozzi G, Dessole M, Catello Di Donna M, Scambia G, et al. Laparoscopic neuronavigation for deep lateral pelvic endometriosis: clinical and surgical implications. J Minim Invasive Gynecol. 2018;25(7):1217-1223.
doi pubmed - Ceccaroni M, Clarizia R, Liverani S, Donati A, Ceccarello M, Manzone M, Roviglione G, et al. Dienogest vs GnRH agonists as postoperative therapy after laparoscopic eradication of deep infiltrating endometriosis with bowel and parametrial surgery: a randomized controlled trial. Gynecol Endocrinol. 2021;37(10):930-933.
doi pubmed - Szubert M, Zietara M, Suzin J. Conservative treatment of deep infiltrating endometriosis: review of existing options. Gynecol Endocrinol. 2018;34(1):10-14.
doi pubmed - Siquara De Sousa AC, Capek S, Amrami KK, Spinner RJ. Neural involvement in endometriosis: Review of anatomic distribution and mechanisms. Clin Anat. 2015;28(8):1029-1038.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, including commercial use, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.