| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://jcgo.elmerpub.com |
Original Article
Volume 14, Number 4, December 2025, pages 155-160
Role of Vaginal Bromocriptine in Symptomatic Management of Adenomyosis
Mita Mandala, d, Subhankar Sarkarb, Subrat Pandaa, Vineet Kumar Kamalc
aDepartment of Obstetrics & Gynecology, AIIMS, Kalyani, India
bDepartment of Paediatrics, AIIMS, Kalyani, India
cDepartment of Biostatistics, AIIMS, Kalyani, India
dCorresponding Author: Mita Mandal, Department of Obstetrics & Gynaecology, AIIMS, Kalyani, India
Manuscript submitted August 3, 2025, accepted November 3, 2025, published online December 11, 2025
Short title: Vaginal Bromocriptine for Adenomyosis
doi: https://doi.org/10.14740/jcgo1531
| Abstract | ▴Top |
Background: Adenomyosis is a non-malignant uterine condition causing heavy bleeding, pelvic pain, and dysmenorrhea, greatly affecting women’s lives. Although its cause is unclear, prolactin may be involved. Treatments vary, with no standard approach. Recent studies suggest that vaginal bromocriptine, a prolactin blocker, may reduce symptoms and improve quality of life. This was a retrospective, single-center study that evaluated the effectiveness and the psychosocial impact of adenomyosis.
Methods: We retrospectively reviewed reproductive-aged women with adenomyosis, heavy menstrual bleeding (HMB), and dysmenorrhea, all with pictorial blood loss assessment chart (PBLAC) scores > 100, treated with 5 mg vaginal bromocriptine daily. Exclusions included pregnancy, lactation, < 6 months postpartum, fibroids, endometriosis, or prior bromocriptine use. Data collected encompassed demographics, PBLAC scores, numeric pain rating scale (NPRS) for pain assessment, and perceived stress scale (PSS) evaluations from case notes.
Results: Among 52 patients (median age 39, interquartile range (IQR) 36 - 45 years), no side effects were reported with vaginal bromocriptine. The PBLAC scores dropped significantly from 235 (220 - 320) at baseline to 232 (220 - 315) at 3 months (P = 0.806), with continued decline. The NPRS scores and stress levels improved notably by 6 months. Ultrasound at 6 months showed significant improvement in vascularity (P = 0.001), junctional zone (P = 0.045), and myometrial thickening (P = 0.014) in those with lesions.
Conclusion: Vaginal bromocriptine was well tolerated, with significant reductions in pain, bleeding, stress, and sonographic changes in adenomyosis. These results suggest that it could be an effective alternative treatment.
Keywords: Adenomyosis; Heavy menstrual bleeding; Dysmenorrhea; Stress; Bromocriptine
| Introduction | ▴Top |
Adenomyosis is a non-malignant uterine condition where the normal endometrium grows into myometrium, leading to hypertrophy of the surrounding tissue [1]. It primarily manifests with debilitating chronic pelvic pain, heavy menstrual bleeding (HMB), and dysmenorrhea [2, 3]. Adenomyosis collectively contributes to anxiety, depression, and psychosocial stress, affecting various aspects of life for women of reproductive age, including work, family life, and physical activity [2]. While a definitive diagnosis typically relies on histopathological examination, adenomyosis is most often diagnosed noninvasively using transvaginal ultrasonography or magnetic resonance imaging (MRI) [3]. The exact etiology of adenomyosis remains unclear despite various proposed theories [4]. Studies in animals suggest that prolactin may contribute to the development of adenomyosis. Both mouse and bovine models have shown associations between elevated prolactin levels (hyperprolactinemia), heightened expression of prolactin receptors (PRLR) in the myometrium, and the onset of adenomyosis [5, 6]. The precise mechanisms underlying elevated prolactin and PRLR in adenomyosis are still not fully elucidated, although in vitro studies indicate that PRL acts as a smooth muscle mitogen [7]. Elevated levels of PRL have been observed in women diagnosed with adenomyosis [8]. The management of adenomyosis is multifaceted, encompassing medical, surgical, and radiological approaches [9]. A recent pilot study has found that administering bromocriptine vaginally, which acts as a prolactin antagonist, notably reduces bleeding and pain during menstruation, also enhancing the quality of life for women diagnosed with adenomyosis [10]. Tang et al found that bromocriptine treatment has an anti-proliferative effect on the endometrium in women with adenomyosis [11]. Our objective was to evaluate the role of vaginal bromocriptine in managing symptoms and addressing the psychosocial impact of adenomyosis.
| Materials and Methods | ▴Top |
From May 2022 to September 2024, we performed a retrospective study at the All India Institute of Medical Sciences, Kalyani, to examine the effects of intravaginal bromocriptine treatment in women diagnosed with adenomyosis and suffering from HMB and dysmenorrhea. The study included all reproductive-aged women diagnosed with adenomyosis via transvaginal ultrasound (TVS), who had a pictorial blood loss assessment chart (PBLAC) score over 100 and were treated with per-vaginal bromocriptine for symptom relief. Exclusions were made for pregnant women, those postpartum for less than 6 months, lactating mothers, individuals with uterine fibroids or endometriosis, known heart disease, and patients with a history of bromocriptine treatments. Cases with concomitant endometriosis were excluded based on clinical assessment and characteristic sonographic findings (e.g., endometriotic cysts or deep infiltrating lesions) according to morphological uterus sonographic assessment (MUSA) consensus criteria. Heavy menstrual bleeding is defined as a PBLAC score greater than 100. In addition to demographic data, several key measures were collected from patient case notes to assess the severity and impact of symptoms. These included demographic data, PBLAC for assessing menstrual blood loss, numeric pain rating scale (NPRS) for pain, and perceived stress scale (PSS) data collected from case notes [12-14]. An NPRS score of 0 indicates no pain, scores ranging from 1 to 3 indicate mild pain, 4 to 6 indicate moderate pain, and 7 to 10 indicate severe pain. The PSS scores ranging from 0 to 13 indicate low stress, 14 to 26 indicate moderate stress, and 27 to 40 indicate high perceived stress. The MUSA provided direct and indirect evidence to confirm the presence and extent of adenomyosis as indicated by TVS findings [15, 16]. To minimize variability, all scans were performed and independently reviewed by experienced radiologists. Intravaginal bromocriptine was offered to women with symptomatic adenomyosis who had inadequate response, contraindications, or intolerance to conventional hormonal therapy and desired uterine preservation. All selected patients were shown how to insert a bromocriptine tablet (2.5 mg) deep into the vagina once daily and were advised to report any side effects, such as fatigue, dizziness, nausea, or headache. After 1 week, if no side effects were observed, the dose was increased to two tablets (5 mg) per day. On follow-up, patients were re-evaluated every 3 months to assess blood loss, pain, and stress levels. A repeat MUSA was conducted after 6 months of bromocriptine therapy to evaluate any changes. No a priori sample size calculation was performed, as all consecutive eligible patients treated during the study period were included. The study is exploratory and aims to generate preliminary data for future prospective trials. Ethical clearance was granted by the Institutional Ethics Committee (IEC) of AIIMS Kalyani (IEC/AIIMS/Kalyani/certificate/2024/303). To ensure accuracy, all data were entered and verified three times to minimize errors. A P-value of less than 0.05 with a 95% confidence interval (CI) was deemed statistically significant. Data are presented as median (interquartile range (IQR)) and were analyzed using the Wilcoxon signed-rank test for paired non-parametric data. Categorical variables were presented as frequencies and percentages and analyzed using the McNemar test. Statistical analyses were performed with STATA version 17.0 (Stata Corp., College Station, TX, USA).
| Results | ▴Top |
A total of 342 patients suspected of adenomyosis were treated for HMB. The majority of them received nonsteroidal anti-inflammatory drugs (NSAIDs) and hormonal treatment (oral pills or intrauterine device), and only 69 patients received vaginal bromocriptine. Patients were excluded from the analysis because bromocriptine was given without having TVS (n = 9), and follow-up data were missing (n = 8). So, the total data of 52 patients with a median age of 39 (IQR 36 - 45) years were analyzed. None of the patients reported any side effects, such as fatigue, dizziness, nausea, headache, rash, gastrointestinal disturbance, or neurological complaints, during follow-up or difficulties in using bromocriptine vaginally. The baseline characteristics are summarized in Table 1. We obtained follow-up data from 52 patients at 3 months, 52 at 6 months, 50 at 9 months, and 50 at 12 months. Two patients switched to alternative therapies after the sixth month of bromocriptine.
 Click to view | Table 1. Baseline Characteristics of Women With Adenomyosis |
Post-bromocriptine changes in bleeding, pain, and stress
The PBLAC score was not significantly decreased from 235 (220 - 320) at baseline to 232 (220 - 315) (P = 0.806) at 3 months, but showed a declining trend thereafter. There was a notable reduction in HMB percentages starting from the sixth month onward (Table 2 and Fig. 1a). The percentages of HMB in the third, sixth, ninth, and 12th months were 100%, 48%, 47.4%, and 50%, respectively. Before bromocriptine treatment, the majority of our patients experienced severe abdominal pain (92.2%). The NPRS score significantly improved from baseline after 6 months of bromocriptine therapy (Table 2 and Fig. 1b). Initially, the majority of patients reported moderate (88%) to high stress levels (7.8%). After 6 months of treatment, stress levels significantly decreased, which corresponded with notable improvements in pain and bleeding symptoms (Table 2 and Fig. 1c).
 Click to view | Table 2. Follow-Up Data of Bleeding, Pain, and Stress |
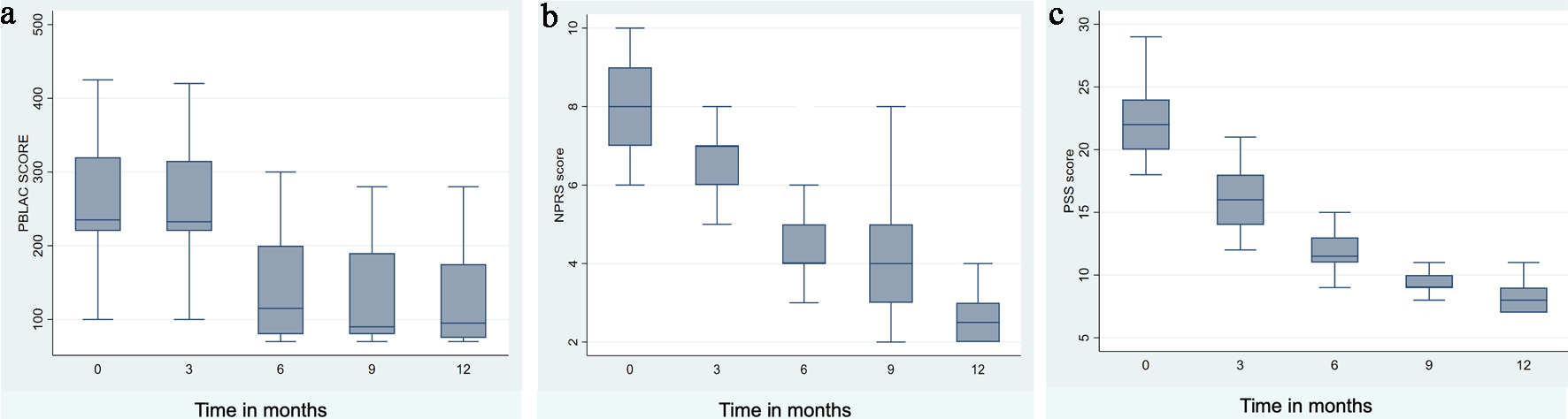 Click for large image | Figure 1. Post-bromocriptine change in bleeding, pain, and stress. |
Post-bromocriptine MUSA changes
The overview of the baseline and changes in MUSA after 6 months of bromocriptine treatment is given in Table 3. Before bromocriptine treatment, the most prevalent sonographic finding was asymmetrical myometrial thickening (78.8%), followed by the presence of translesional vascularity (76.9%). After 6 months of bromocriptine therapy, translesional vascularity decreased from 76.9% to 55.8% (P = 0.001), asymmetrical myometrial thickening decreased from 78.8% to 65.4% (P = 0.016), and junctional zone abnormality decreased from 55.7% to 48.1% (P = 0.045). Other MUSA features showed non-significant trends toward improvement, with no new lesions detected. No new lesions were detected during follow-up.
 Click to view | Table 3. Transvaginal Ultrasound Findings of the Uterus |
| Discussion | ▴Top |
The current study revealed that vaginal bromocriptine significantly reduces menstrual bleeding, pain, and stress in women with adenomyosis. Throughout the treatment, both pain and stress levels remained consistently low. However, significant changes were observed in indirect MUSA features, including translesional vascularity, junctional zone abnormality, and asymmetrical myometrial thickening. In contrast, there was no change in direct features, such as echogenic sub-endometrial lines and buds, hyperechogenic islands, and myometrial cysts. In a pilot study, Anderson et al treated 23 women diagnosed with diffuse adenomyosis using vaginal bromocriptine. They observed significant improvements in abnormal uterine bleeding, dysmenorrhea, and overall quality of life following 6 months of treatment [10]. Subsequently, the researchers conducted another study focusing on the impact of vaginal bromocriptine on MRI and vaginal ultrasound findings in adenomyosis patients, particularly those with enlarged uterine size and asymmetrical uterine walls. In this study, 18 women were followed up for 6 months, during which significant improvements in symptoms, along with a notable reduction in uterine size and an enhancement in uterine morphology, were observed. These findings suggest that vaginal bromocriptine may offer effective therapeutic benefits for women with adenomyosis, especially those with more severe cases [17]. Compared with the two studies by Andersson et al, which included 23 and 18 patients, respectively, the present study comprised a larger cohort of 52 patients. Despite its retrospective design, our findings are consistent with these earlier reports.
Several treatment options for adenomyosis have been proposed, but definitive management guidelines are currently lacking. While hysterectomy remains the definitive treatment, conservative options for patients seeking to preserve fertility are limited by insufficient high-quality data. Tranexamic acid and NSAIDs play limited roles in therapy, primarily providing relief for mild symptoms or symptom flares during hormone therapy. Among the available therapy options, the levonorgestrel intrauterine device (LNG-IUD) has proven to be the most effective [18]. Other treatments, including gonadotropin-releasing hormone (GnRH) antagonists, dienogest, and modulators of prolactin and oxytocin, hold potential but require further research to validate their efficacy [19]. It is noteworthy that the use of hormonal contraceptives and LNG-IUDs is linked to a 13% increased risk of breast cancer [20]. In addition to medical and surgical therapy, radiological procedures such as uterine artery embolization and high-intensity focused ultrasound have emerged as effective minimally invasive options for symptom relief and uterine volume reduction [9].
Our approach involved vaginal administration of bromocriptine to minimize systemic side effects and achieve a concentrated impact on the uterus. Previous studies have shown that this method reduces gastrointestinal side effects compared to oral administration [21]. The use of bio-adhesive bromocriptine mesylate vaginally effectively lowers serum prolactin levels in hyperprolactinemia patients, demonstrating significant results within a shorter treatment timeframe [22]. Bromocriptine was well tolerated by our cohort; however, long-term use should be individualized, with regular monitoring of efficacy, tolerability, and cardiovascular safety, especially in perimenopausal women.
Our study, despite its limitations, including small sample size, single-center design, and retrospective nature, offers valuable insights into the combined clinical and radiological outcomes of bromocriptine in a uniform group of women with adenomyosis. The present study included a relatively large cohort of 52 patients, among the largest reported to date. The absence of a control group (placebo or standard therapy such as LNG-IUD) limits the ability to attribute symptom improvement solely to bromocriptine. Moreover, the small cohort may not capture rare adverse effects or variability in response. The 12-month follow-up may also be insufficient to assess long-term efficacy, recurrence, or fertility outcomes, as symptoms of adenomyosis often fluctuate over time. Moreover, MUSA features were recorded as binary, which may overlook partial changes. These insights emphasize the growing need for effective, minimally invasive treatment[ options for adenomyosis. Multicenter studies with extended follow-up periods are needed to further elucidate bromocriptine’s efficacy in treating adenomyosis. Such research efforts are crucial for optimizing treatment strategies and improving care for women affected by this condition.
Conclusion
Vaginal bromocriptine was well tolerated in our cohort, demonstrating significant reductions in pain, bleeding, and stress levels, alongside notable improvements in sonographic indicators of adenomyosis. These findings underscore the therapeutic potential of bromocriptine in managing symptoms of adenomyosis. As a result, it may serve as a valuable alternative for effectively treating adenomyosis.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
This study is based on a review of case files and, therefore, the need for consent from individual women was waived. The facility’s consent to be part of the study was signed by the Head of the Department.
Author Contributions
Conception or design of the work: MM, SS, and SP; data collection: MM and SS; data analysis and interpretation: MM, SS, and VKKSG; drafting the article: MM, SS, and VKK; critical revision of the article: SS and SP. All authors read and approved the manuscript for publication.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Aleksandrovych V, Basta P, Gil K. Current facts constituting an understanding of the nature of adenomyosis. Adv Clin Exp Med. 2019;28(6):839-846.
doi pubmed - Martone S, Centini G, Exacoustos C, Zupi E, Afors K, Zullo F, Maneschi F, et al. Pathophysiologic mechanisms by which adenomyosis predisposes to postpartum haemorrhage and other obstetric complications. Med Hypotheses. 2020;143:109833.
doi pubmed - Cockerham AZ. Adenomyosis: a challenge in clinical gynecology. J Midwifery Womens Health. 2012;57(3):212-220.
doi pubmed - Zhai J, Vannuccini S, Petraglia F, Giudice LC. Adenomyosis: mechanisms and pathogenesis. Semin Reprod Med. 2020;38(2-03):129-143.
doi pubmed - Lupicka M, Socha BM, Szczepanska AA, Korzekwa AJ. Prolactin role in the bovine uterus during adenomyosis. Domest Anim Endocrinol. 2017;58:1-13.
doi pubmed - Singtripop T, Mori T, Park MK, Sakamoto S, Kawashima S. Development of uterine adenomyosis after treatment with dopamine antagonists in mice. Life Sci. 1991;49(3):201-206.
doi pubmed - Yamashita M, Matsuda M, Mori T. Increased expression of prolactin receptor mRNA in adenomyotic uterus in mice. Life Sci. 1997;60(17):1437-1446.
doi pubmed - Stratopoulou CA, Donnez J, Dolmans MM. Origin and pathogenic mechanisms of uterine adenomyosis: what is known so far. Reprod Sci. 2021;28(8):2087-2097.
doi pubmed - Capezzuoli T, Toscano F, Ceccaroni M, Roviglione G, Stepniewska A, Fambrini M, Vannuccini S, et al. Conservative surgical treatment for adenomyosis: new options for looking beyond uterus removal. Best Pract Res Clin Obstet Gynaecol. 2024;95:102507.
doi pubmed - Andersson JK, Khan Z, Weaver AL, Vaughan LE, Gemzell-Danielsson K, Stewart EA. Vaginal bromocriptine improves pain, menstrual bleeding and quality of life in women with adenomyosis: A pilot study. Acta Obstet Gynecol Scand. 2019;98(10):1341-1350.
doi pubmed - Tang Y, Ponandai-Srinivasan S, Frisendahl C, Andersson JK, Pavone D, Stewart EA, Lalitkumar PGL, et al. Bromocriptine inhibits proliferation in the endometrium from women with adenomyosis. Front Endocrinol (Lausanne). 2023;14:1026168.
doi pubmed - Higham JM, O'Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol. 1990;97(8):734-739.
doi pubmed - McCaffery M, Beebe A. Pain: clinical manual for nursing practice. St. Louis, MO: Mosby; 1989.
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
pubmed - Van den Bosch T, Dueholm M, Leone FP, Valentin L, Rasmussen CK, Votino A, Van Schoubroeck D, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015;46(3):284-298.
doi pubmed - Harmsen MJ, Van den Bosch T, de Leeuw RA, Dueholm M, Exacoustos C, Valentin L, Hehenkamp WJK, et al. Consensus on revised definitions of Morphological Uterus Sonographic Assessment (MUSA) features of adenomyosis: results of modified Delphi procedure. Ultrasound Obstet Gynecol. 2022;60(1):118-131.
doi pubmed - Andersson JK, Pozzi Mucelli R, Epstein E, Stewart EA, Gemzell-Danielsson K. Vaginal bromocriptine for treatment of adenomyosis: Impact on magnetic resonance imaging and transvaginal ultrasound. Eur J Obstet Gynecol Reprod Biol. 2020;254:38-43.
doi pubmed - Moawad G, Youssef Y, Fruscalzo A, Faysal H, Kheil M, Pirtea P, Guani B, et al. The present and the future of medical therapies for adenomyosis: a narrative review. J Clin Med. 2023;12(19):6130.
doi pubmed - Etrusco A, Barra F, Chiantera V, Ferrero S, Bogliolo S, Evangelisti G, Oral E, et al. Current medical therapy for adenomyosis: from bench to bedside. Drugs. 2023;83(17):1595-1611.
doi pubmed - Morch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard O. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228-2239.
doi pubmed - Kletzky OA, Vermesh M. Effectiveness of vaginal bromocriptine in treating women with hyperprolactinemia. Fertil Steril. 1989;51(2):269-272.
doi pubmed - Darwish AM, Farah E, Gadallah WA, Mohammad II. Superiority of newly developed vaginal suppositories over vaginal use of commercial bromocriptine tablets: a randomized controlled clinical trial. Reprod Sci. 2007;14(3):280-285.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.