| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://jcgo.elmerpub.com |
Case Report
Volume 14, Number 1, January 2025, pages 31-38
Pregnancy Complicated by Complete Atrioventricular Block
Yuda Putra Disastraa , Dalri M. Suhartomob, d
, Wiryawan Permadia, Abraham Avicennac
aDepartment of Obstetrics and Gynecology, Faculty of Medicine, Universitas Padjadjaran-Dr. Hasan Sadikin General Hospital, Bandung, Indonesia
bDepartment of Obstetrics and Gynecology, Faculty of Medicine, Universitas Jenderal Soedirman-Prof. Dr. Margono Soekarjo General Hospital, Purwokerto, Indonesia
cDepartment of Cardiology, Prof. Dr. Margono Soekarjo General Hospital, Purwokerto, Indonesia
dCorresponding Author: Dalri M. Suhartomo, Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Jenderal Soedirman-Prof. Dr. Margono Soekarjo General Hospital, Purwokerto, Indonesia
Manuscript submitted October 25, 2024, accepted January 10, 2025, published online January 24, 2025
Short title: Pregnancy Complicated by CAVB
doi: https://doi.org/10.14740/jcgo1009
| Abstract | ▴Top |
Bradycardia in pregnancy resulting from complete atrioventricular block (CAVB) is a rare but critical condition that can threaten both maternal and fetal health. If not managed appropriately, it can lead to severe complications, emphasizing the need for timely diagnosis and intervention. The incidence of CAVB during pregnancy is approximately 1 in 15,000 to 20,000 pregnancies. Most cases present clinically with symptoms such as syncope or palpitations, highlighting the importance of prompt recognition and management. While some patients with CAVB may be asymptomatic, symptomatic cases require urgent and definitive management. There is currently no established guideline for managing CAVB during pregnancy. We report the case of a 20-year-old primigravida at 35 weeks and 5 days of gestation, who presented with heart conduction problems (i.e., bradycardia) and arrived at the obstetric emergency department after receiving magnesium sulfate for seizure prophylaxis due to her high-risk profile and two intravenous doses of atropine sulfate. The patient experienced seizures and worsening bradycardia, prompting an emergency cesarean section. A female infant was delivered with a birth weight of 2,525 g, a length of 45 cm, and a New Ballard Score corresponding to gestational age of 36 weeks. No congenital anomalies were identified. The patient had an uncomplicated recovery and was discharged with a cardiologist’s recommendation for follow-up echocardiography. CAVB in pregnancy can arise from either congenital or acquired conditions, both of which may lead to maternal cardiovascular decompensation and fetal complications. In such cases, pacemaker implantation is often required to manage symptomatic bradycardia, and cesarean section may be the preferred mode of delivery to ensure both maternal and fetal safety. This case highlights the critical need for timely and appropriate management of symptomatic CAVB during pregnancy. Pacemaker implantation serves as the definitive treatment option for pregnant patients with CAVB, effectively addressing bradycardia and preventing further complications.
Keywords: Bradycardia; Cesarean section; Complete atrioventricular block; Eclampsia
| Introduction | ▴Top |
The epidemiology of complete atrioventricular block (CAVB) in pregnancy within Asia is not extensively documented, one report indicated that the prevalence of atrioventricular (AV) block during pregnancy is approximately 11.28% among patients with cardiac arrhythmias [1]. CAVB is a condition characterized by a disruption in the heart’s electrical conduction system, resulting in the complete failure of electrical impulses to be transmitted from the atria to the ventricles. This is classified as third-degree AV block and can lead to significant bradycardia and other complications if not addressed promptly [2-4]. CAVB is generally classified into two categories: congenital and acquired. Congenital CAVB often arises in the context of maternal autoimmune diseases, where antibodies can interfere with normal heart development in the fetus. Acquired CAVB may result from several factors, including cardiac surgery, infections and autoimmune diseases, which can disrupt the electrical conduction system of the heart [2, 4, 5]. CAVB can lead to severe complications, including an increased risk of Stokes-Adams syndrome, ventricular tachycardia, syncope and potentially sudden death. The incidence of CAVB during pregnancy is rare, estimated at 1 in 15,000 to 20,000 pregnancies. Most cases present clinically with symptoms such as syncope, which results from cerebral hypoperfusion and palpitations [6, 7]. CAVB is caused by congenital structural abnormalities of the heart in approximately 50-55% of cases. Among these, an estimated 40% are associated with maternal antibodies, specifically those that are positive for Sjogren’s syndrome antigen A (SSA/Ro) or Sjogren’s syndrome antigen B (SSB/La). These antibodies can cross the placenta and disrupt normal fetal cardiac development, leading to the occurrence of CAVB in newborns [8, 9]. The antibodies will pass through the placental circulation and cause immune-mediated inflammation or fibrosis in the fetal heart conduction tissue [8-10]. CAVB is characterized by AV dissociation and low ventricular heart rate (pulse rate < 60 beats per minute (bpm)) due to lack of conduction between atrium and ventricle leading to decreased perfusion and resulting in symptoms of bradycardia, low cardiac output (CO) and arrhythmia [1, 6]. Currently, there is no consensus regarding the management of CAVB during pregnancy, making it a topic of ongoing discussion among obstetricians, cardiologists, and anesthesiologists. Treatment considerations vary based on antenatal, intrapartum, and postpartum conditions, as well as the selection of appropriate contraceptive methods for affected patients. As the clinical landscape evolves, further research is needed to establish standardized protocols for the management of CAVB in pregnant patients. This case report has been prepared in accordance with the Case Report (CARE) guidelines, including its purpose and significance in case reporting.
| Case Report | ▴Top |
A 20-year-old primigravida at 35 weeks and 5 days of gestation was referred to the emergency department by her obstetrician, diagnosed with severe preeclampsia, bradycardia suspected to be due to CAVB, and ventricular extrasystoles. During the referral journey, the patient experienced a seizure lasting less than 1 min but regained consciousness afterward. Her medical history was evaluated according to the Framingham criteria, and she denied any previous seizures. Since 34 weeks of gestation, the patient had been experiencing high blood pressure, recorded at 173/100 mm Hg, without any severe features indicative of worsening preeclampsia. The referring hospital had initiated treatment with magnesium sulfate (MgSO4) for seizure prophylaxis, alongside a maintenance dose, and administered two ampules of atropine sulfate intravenously to address bradycardia.
In the emergency management room, the patient’s blood pressure was recorded at 133/80 mm Hg, with a pulse of 36 bpm and a respiratory rate of 20 breaths per minute. The electrocardiogram (ECG) revealed CAVB with an escape ventricular rhythm (Fig. 1). The patient was unaware of any prior heart conduction issues before her pregnancy. We had elaborated on the differential diagnosis process, in which peripartum cardiomyopathy was excluded through clinical assessment such as normal left ventricular function, absence of systolic dysfunction, ECG findings and echocardiography, all of which are critical in differentiating peripartum cardiomyopathy. During her time in the emergency obstetrics unit, the patient’s condition deteriorated, prompting a referral to the resuscitation room due to the onset of pulseless ventricular tachycardia lasting less than 2 min, followed by asystole. Cardiopulmonary resuscitation (CPR) was initiated. Upon regaining consciousness, the obstetrician decided to proceed with an urgent cesarean section and intrauterine device (IUD) insertion. A female infant was delivered, weighing 2,525 g and measuring 45 cm in length, with no congenital anomalies detected. Post-delivery, the patient was closely monitored in the intensive care unit (ICU) until her condition stabilized before being transferred to the general obstetric ward. There were no further complications experienced by the patient following discharge.
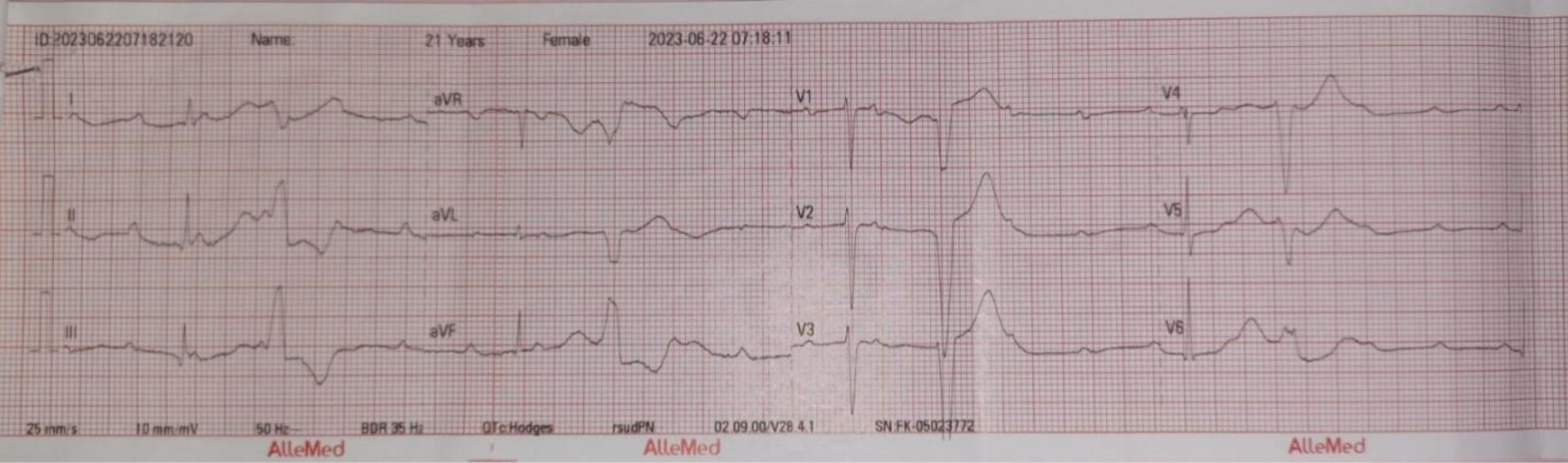 Click for large image | Figure 1. Electrocardiogram pre-delivery: third-degree AV block with ventricular escape on June 22, 2023. AV: atrioventricular. |
Investigation
Postoperative chest X-ray revealed cardiomegaly, characterized by left ventricular and left atrial hypertrophy (Fig. 2); however, the lungs appeared normal. Routine laboratory tests, including electrolyte levels (e.g., sodium, potassium, calcium, and chloride), returned normal results. Unfortunately, magnesium levels could not be assessed due to limited facility resources. An ECG confirmed the presence of CAVB with an escape ventricular rhythm, without signs of ischemic changes (Fig. 3). An echocardiogram was scheduled for follow-up after outpatient assessment.
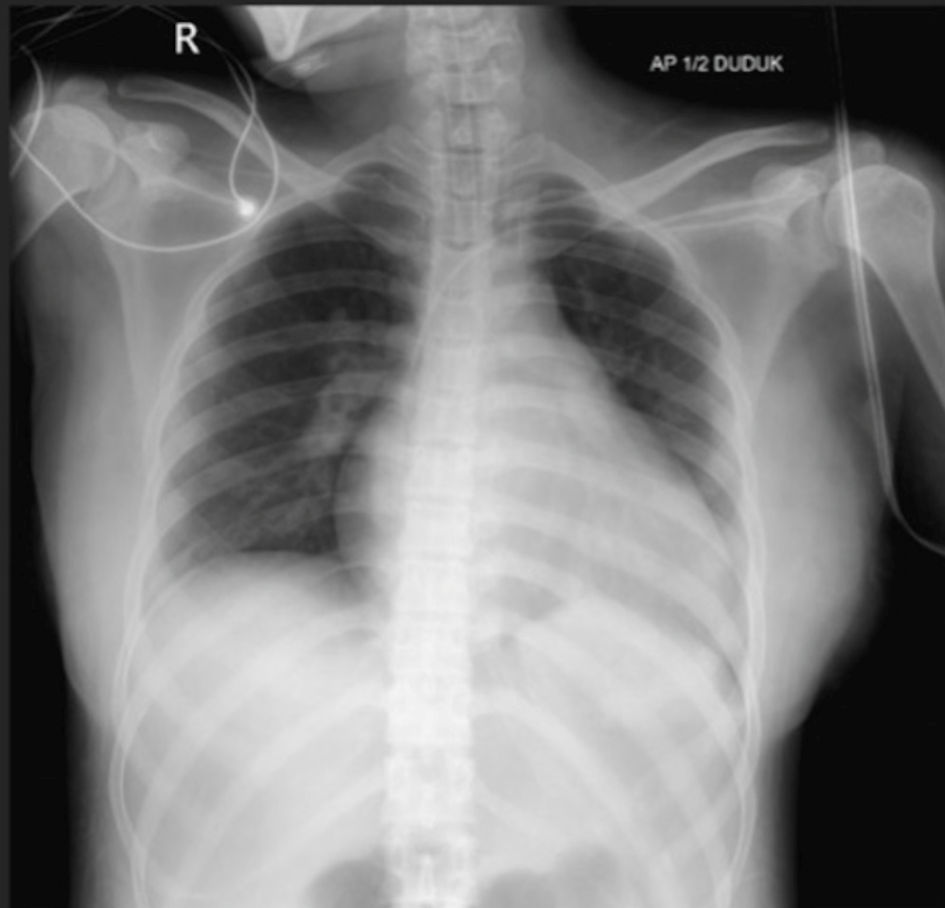 Click for large image | Figure 2. Post-cesarean section chest X-ray: cardiomegaly characterized by left ventricular and left atrial hypertrophy on June 23, 2023. |
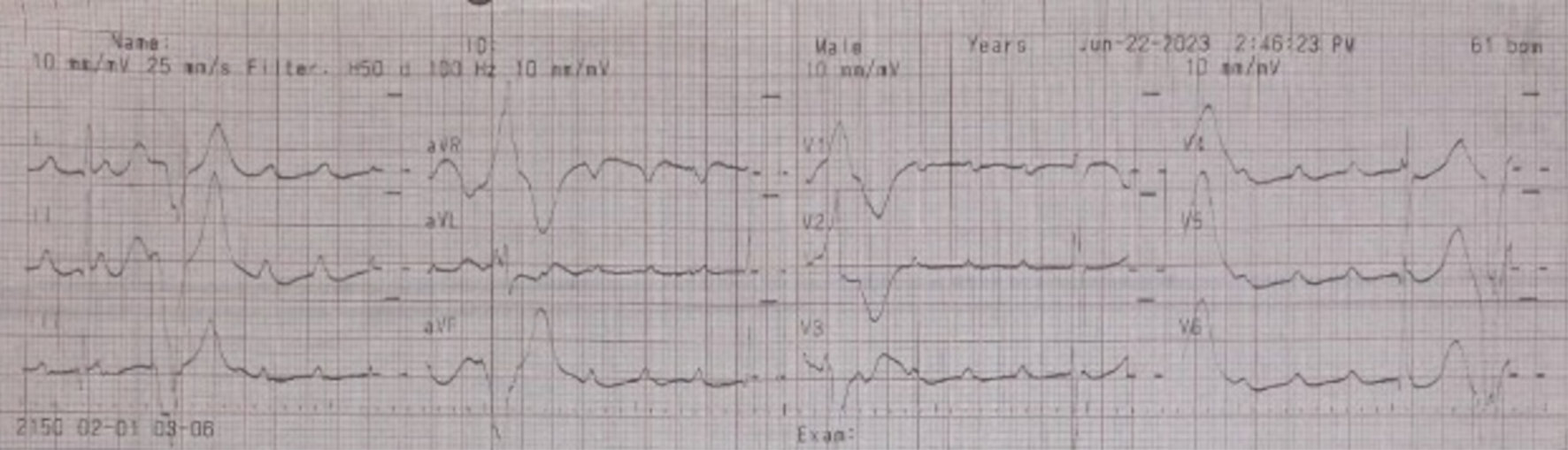 Click for large image | Figure 3. Electrocardiogram post-cesarean section: third-degree AV block with ventricular escape on June 22, 2023. AV: atrioventricular. |
Differential diagnosis
The differential diagnosis included eclampsia and severe eclampsia, as determined by physical examination and history taking, along with sinus bradycardia, as indicated by typical ECG findings.
Management
The obstetrician decided to perform an urgent cesarean section due to eclampsia, with consultations from a cardiologist and anesthesiologist regarding the patient’s management during and after the operation. Anesthesia recommendations included postoperative monitoring in the ICU, an ECG on postoperative day (POD) 1 (Fig. 4), and the administration of midazolam (1 mg/h) and morphine (1 mg/h) via a syringe pump until POD 1. Additionally, dopamine was titrated down to 5 µg/h by POD 1, prior to extubation.
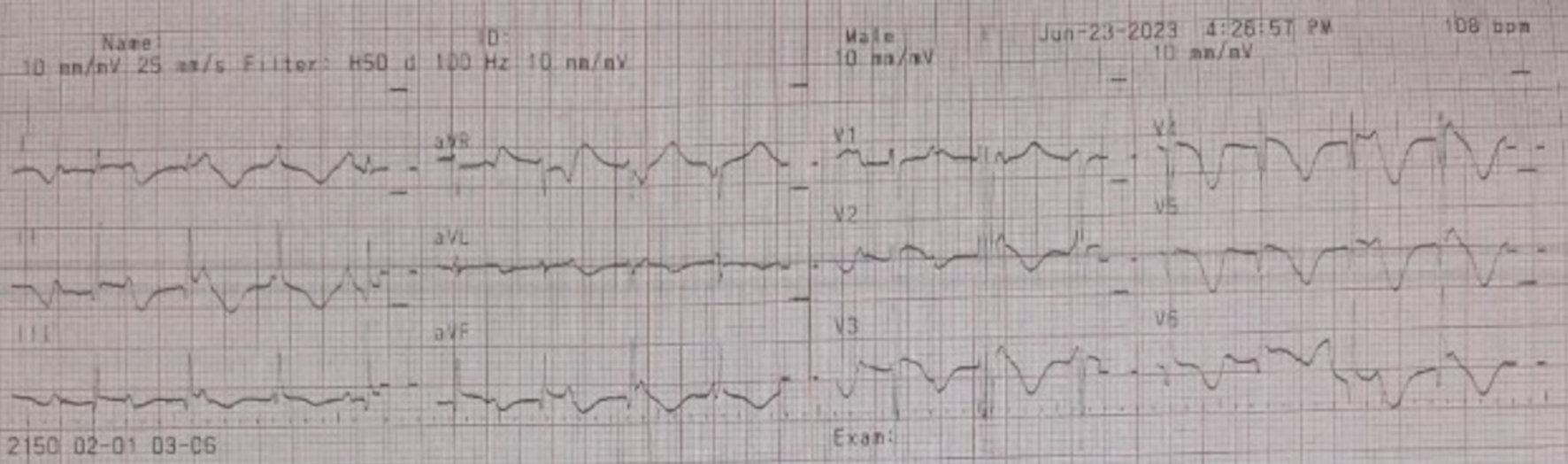 Click for large image | Figure 4. Electrocardiogram post-cesarean section (POD 1): third-degree AV block with ventricular escape on June 23, 2023. POD: post-operation day; AV: atrioventricular. |
The patient was observed in the ICU for 2 days, during which her condition continued to improve. The cardiologist recommended administering 1 mg of atropine sulfate intravenously three times while awaiting surgery. The treatment approach focused on monitoring the progression of bradycardia, with the option to implant a pacemaker at a specialized cardiology center. However, as the patient’s condition improved significantly, her family declined the pacemaker implantation. The cardiologist recommended scheduling an echocardiogram for follow-up after the outpatient assessment.
Follow-up and outcomes
A term female infant was delivered via cesarean section on Thursday, May 22, 2023, at 12:40 pm. At birth, the baby weighed 2,525 g, measured 45 cm in length, and had a head circumference of 32 cm and a chest circumference of 30 cm. The infant’s Apgar (appearance, pulse, grimace, activity, respiration) scores were 5 at 1 min, 6 at 5 min, and 7 at 10 min, with a New Ballard score of 30, indicating a gestational age of approximately 36 weeks. No congenital anomalies were detected, and the baby was monitored in the perinatology unit. Both the mother and baby were discharged in stable condition, with the mother scheduled for a follow-up appointment at the cardiology clinic 1 week later. An echocardiogram was arranged for October 12, 2023. The echocardiogram findings revealed the following: left ventricular dilation, eccentric left ventricular hypertrophy, no thrombus or pericardial effusion, an intact septum and global normokinesis of the wall motion. Left ventricular contractility was assessed as grade III diastolic dysfunction, while right ventricular contractility was good. Mild mitral valve regurgitation and mild tricuspid valve regurgitation were observed, with the aortic valve appearing normal. The final conclusion was left ventricular hypertrophy, grade III diastolic dysfunction of the left ventricle, normal chamber dimensions, preserved left ventricular ejection fraction and mild mitral and tricuspid regurgitation (Fig. 5).
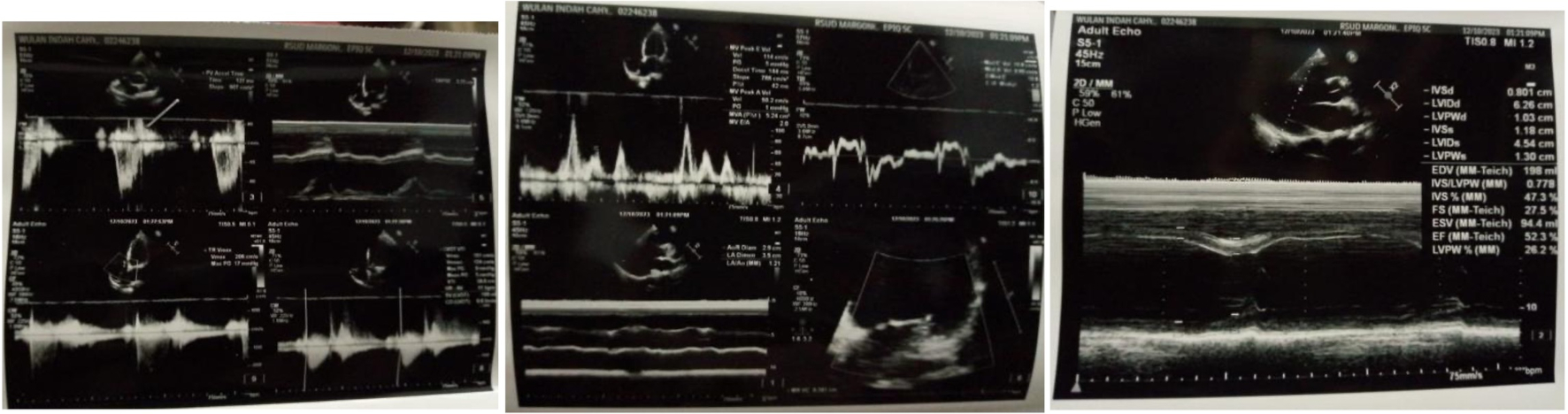 Click for large image | Figure 5. Echocardiography examination on October 12, 2023. |
| Discussion | ▴Top |
In pregnancy, physiological adaptations occur in the cardiovascular system, including significant changes in CO, which increases by approximately 30% as early as 5 weeks of gestation [11]. This increase in CO is accompanied by a decrease in systemic vascular resistance and an elevation in heart rate. These adaptations are essential for meeting the heightened metabolic demands of both the mother and the developing fetus [4, 12]. Normal CO typically increases during the first and second trimesters of pregnancy to accommodate the heightened metabolic demands associated with gestation. This increase is primarily driven by enhanced blood volume and cardiac efficiency, which ensures adequate perfusion to both the mother and the developing fetus. The body’s adaptations are crucial for supporting the growing needs of the placenta and fetal tissues while maintaining maternal health [6, 13]. An increase in CO is primarily achieved when stroke volume (SV) is adequate. SV refers to the amount of blood ejected by the heart with each contraction. During pregnancy, physiological adaptations, such as increased blood volume and enhanced venous return, contribute to improved SV, thereby facilitating the rise in CO required to meet the metabolic demands of both the mother and the fetus [1]. These changes will manifest as ECG alterations, such as slight left axis deviation resulting from positional changes of the heart during pregnancy. Additionally, Q waves may be observed in leads II, III, and augmented vector foot (aVF), along with flattened or inverted T waves in leads III and V1 - V3. These findings can be attributed to the physiological adaptations of the cardiovascular system that occur during pregnancy [5, 13]. In pathological conditions, the electrical activity of the heart can significantly impact heart rhythm, leading to various arrhythmias. Common manifestations include bradyarrhythmia, supraventricular tachycardia (SVT), ventricular tachycardia and prolonged QT interval. These arrhythmias can arise from various factors, including structural heart disease, electrolyte imbalances, and medication effects, and pose significant risks for morbidity and mortality if not appropriately managed [6, 8]. Bradyarrhythmia, characterized by a pulse rate of less than 60 bpm, can occur in patients with CAVB during pregnancy or childbirth, often manifesting as syncope. In such cases, temporary cardiac pacing or the use of an external pacemaker may be necessary to stabilize the heart rate. This intervention aims to improve CO by enhancing SV, thus ensuring adequate perfusion to both the mother and the fetus [6, 13].
CAVB can be divided into two categories: 1) Congenital CAVB is often associated with maternal autoimmune diseases, particularly in women with anti-Sjogren’s syndrome-related antigen A (anti-SSA) and anti-Sjogren’s syndrome-related antigen B (anti-SSB) or anti-La antibodies. These antibodies can cross the placental barrier, leading to immune-mediated injury to the conduction system of the fetus and neonate. CAVB associated with autoimmune conditions is typically diagnosed during adolescence. Other autoimmune diseases linked to CAVB include ankylosing spondylitis and systemic sclerosis [3, 8]. Approximately 30% of cases of congenital CAVB remain undiagnosed until adulthood, and symptoms may manifest during pregnancy. This delayed recognition can complicate the management of cardiac conditions in pregnant individuals, particularly as the physiological changes associated with pregnancy can exacerbate underlying cardiac issues [14]. 2) Acquired disorders that can lead to CAVB include several cardiovascular and infectious conditions. These may include coronary artery disease following myocardial infarction, non-ischemic cardiomyopathy potentially caused by digoxin toxicity, and complications from heart surgery, particularly mitral valve replacement. Additionally, myocarditis resulting from viral infections or giant cell infiltration can contribute to CAVB. Infectious causes include infective endocarditis, especially when associated with paravalvular abscess formation, as well as diseases such as Lyme disease, Chagas disease (trypanosomiasis), and infections due to Aspergillus species and varicella zoster virus [3, 8].
The diagnosis of CAVB can be established through a 12-lead ECG. Typically, the rate of P waves is faster than that of QRS complexes. In CAVB, an escape rhythm ensures that the RR intervals are often regular, while AV dissociation leads to fluctuations in the PR intervals. Both narrow QRS complexes, which may arise from junctional escape rhythms occurring above the bundle branches, and wide QRS complexes, which may indicate ventricular escape or junctional escape with a bundle branch block, can be observed [6]. In this case report, the ECG revealed CAVB accompanied by ventricular escape rhythms. This finding is characteristic of CAVB, where the atria and ventricles beat independently, often resulting in a slower escape rhythm from the ventricles to maintain some degree of CO. Ventricular escape rhythms can be identified by their wider QRS complexes, which may vary depending on the location of the escape pacemaker (either junctional or ventricular).
MgSO4 loading and maintenance dosages were initially administered to the referring patients; however, at the obstetric emergency room, the administration of MgSO4 was discontinued. This decision was made due to concerns that MgSO4 could exacerbate maternal cardiac muscle contractility issues in patients with bradycardia and CAVB [15, 16]. Elevated magnesium levels can potentially lead to further depression of cardiac function, particularly in individuals already experiencing compromised CO [17]. Peripheral blood vessels may experience diminished benefits from MgSO4 administration due to its potential to reduce cardiac contractility and its negative inotropic effects. Consequently, the administration of this drug is generally not advised for patient populations at risk of cardiac complications, particularly those with bradycardia and CAVB [16, 17]. In such cases, the risks may outweigh the benefits, resulting in healthcare providers exercising caution in its use. When CO is stable, the administration of magnesium at specific levels can lead to a decrease in vascular resistance, arterial pressure and heart rate. Cardiac performance is determined by several factors, including heart rate, SV and myocardial oxygen consumption. These elements interact simultaneously with a reduction in vascular resistance, which can contribute to improved hemodynamic stability.
Pregnancy outcome
Numerous studies indicate that fetal complications in pregnant women with CAVB can include prematurity, oligohydramnios, small gestational age, and in some cases, fetal distress. Additionally, maternal complications associated with CAVB may encompass premature rupture of membranes (PROM), gestational diabetes, preeclampsia, placental adhesion, velamentous placenta, thrombocytopenia, proteinuria and edema [5, 9]. In this case, the complication that occurred was premature labor; however, it was not accompanied by small gestational age or intrauterine growth restriction. This highlights that while CAVB can lead to various adverse outcomes during pregnancy, not all cases present with the same complications. This indicates the importance of individualized monitoring and management for pregnant patients with this condition.
Delivery options
The mode of delivery in cases of CAVB during pregnancy is primarily guided by obstetric indication [5, 7]. Sullivan et al conducted a study that advocated for cesarean section due to fetal indications, while Mandal et al reported cases where vaginal delivery was proposed [18, 19]. These differing recommendations highlight the complexity of managing delivery methods in cases of CAVB during pregnancy, emphasizing the need for individualized assessment based on maternal and fetal conditions [20]. The augmentation of labor to shorten the active phase and the use of elective instrumental delivery to expedite the second stage have been recommended for women with CAVB. This is particularly important as these women are susceptible to syncopal attacks and seizures, which can occur due to bradycardia associated with the Valsalva maneuver during forceful uterine contractions. It is crucial to consider these factors when planning delivery to ensure the safety of both the mother and fetus [5, 7, 20].
Indications for pacemaker implantation
In patients presenting with a history of syncope, a wide QRS complex, signs of heart failure, prolonged QT interval and a very low heart rate (below 40 bpm), a pacemaker is often recommended. This intervention is critical in preventing serious complications such as syncope, cardiac arrest, or sudden cardiac death [2, 6]. Implanting a permanent pacemaker during pregnancy is generally considered safe, but timing and clinical considerations are crucial. Permanent pacemaker implantation can be performed at any point during pregnancy; however, it is recommended to wait until after the first trimester (typically after 8 weeks) to minimize risks to the developing fetus. Preferably, the second trimester is chosen due to reduced risks of miscarriage [2, 3, 6]. The main points of controversy include the necessity of replacing the generator, the exposure to teratogenic fluoroscopic materials prior to pacemaker implantation and the challenges that arise after the implantation [2]. According to Hidaka et al, women with CAVB who do not have a permanent pacemaker typically do not require temporary pacing during labor and delivery [21]. This observation suggests that in the absence of significant symptoms, such as severe bradycardia or syncope, many patients can manage labor without the need for additional pacing support. This finding aligns with the understanding that while CAVB can present challenges during pregnancy and delivery, many patients can successfully undergo labor without the risks associated with temporary pacing [20]. According to the European Society of Cardiology (ESC) Guidelines, isolated congenital atrioventricular (iAV) block, particularly when associated with a narrow QRS complex, generally shows an improved prognosis during pregnancy [22]. In stable patients, the guidelines do not recommend temporary pacing unless the patient exhibits symptoms related to syncope or significant bradycardia. This reflects the understanding that well-managed cases can navigate pregnancy without the added risks associated with temporary pacing interventions. These guidelines highlight the importance of individualized care based on the patient’s clinical condition, emphasizing that careful monitoring and symptom management are key during pregnancy [22, 23].
Monitoring
Antenatal
Before discussing antenatal care in CAVB during pregnancy, patients should be monitored closely and counseled prior to getting pregnant. The family should also be informed about the possibility of needing a pacemaker during pregnancy [6, 14]. To determine whether to use an external pacemaker or medication, a cardiology examination is required if the patient develops symptoms such as syncopal attacks and dyspnea during prenatal care [2, 7].
Intrapartum
Patients need to be assessed and closely monitored for proper analgesia and an ECG should be taken to identify potentially fatal bradycardia [2, 3]. Lower segment cesarean section (LSCS) may be indicated based on obstetric indications; instrumental delivery can be performed in the second stage of operative vaginal delivery, especially in exceptional circumstances where the pregnant woman’s heart evaluation is inadequate [7, 20]. In the event of an emergency requiring transvenous temporary pacing, the femoral or jugular venous access method must be readily available while isoprenaline infusion is on standby. In the event that transcutaneous temporary pacing is available, the pads should be fastened to the chest wall to facilitate prompt installation of the pacemaker [4].
Postpartum
In order to prevent asystole and postpartum cardiac arrest, patients with post-bradycardia disorders should undergo a heart examination [24, 25]. Patients who require a permanent pacemaker due to CAVB should have routine follow-up for ECG or echocardiography every 6 months [5, 6]. The patient’s history of heart conduction abnormalities (e.g., CAVB), the need for breastfeeding, the time elapsed since birth, the desire to have another child, the delay in pregnancy following a cesarean section and other factors are all taken into consideration [3, 10, 20]. Although AV block is considered category 2 according to the World Health Organization (WHO) Medical Eligibility Criteria (MEC) for contraception, all combined hormonal contraceptive methods, including implants and IUDs (e.g., copper-IUD or levonorgestrel-IUD) are considered safe; breastfeeding mothers who are 48 h or less postpartum may also use an IUD (MEC category 2), and combined contraception is not contraindicated [26]. The family has received contraceptive counseling and, as a result, decided to have an IUD implanted.
Anesthesiologist interventions
The appropriate anesthetic dosage for a patient with CAVB is debatable due to the possibility of blockage at high sympathetic levels, which could result in fatal bradycardia; therefore, spinal anesthesia is not recommended. Meanwhile, under general anesthesia, some problems like bradycardia, hypertension, arrhythmia and cardiac arrest may occur [13, 27]. Hemodynamics can also be significantly affected by inhaled gases and injected medicines. In general, anesthesia drugs that have the least depressive effect on heart rate, such as bupivacaine, fentanyl, ketamine, pancuronium and isoflurane, which do not generate bradycardia, are preferred [13]. In this case, the patient received 100 µg of fentanyl, 100 mg of propofol and 50 mg of rocurium for general anesthesia, followed by intubation. After surgery, the patient was observed in the ICU. Dopamine was administered and gradually tapered off to 5 µg/h. The patient was extubated on POD 1 and transferred to the obstetric ward on POD 2.
Cardiologist interventions
According to the 2018 guidelines from the American College of Cardiology on the management of patients with bradycardia and cardiac conduction delay, the bradycardia management algorithm revealed the need to identify the etiology of bradycardia, due to AV block, and then proceed with acute management. This may include atropine (0.5 - 1 mg intravenous (IV), which may be repeated every 3 - 5 min to a maximum dose of 3 mg), and dopamine (5 - 20 µg/kg/min IV, starting at 5 µg/kg/min, and increased by 5 µg/kg/min every 2 min). It is important to be cautious, as dosages of > 20 µg/kg/min may result in vasoconstriction or arrhythmias. Isoproterenol may be used at a dose of 20 - 60 µg IV bolus, followed by doses of 10 - 20 µg or infusion of 1 - 20 µg/min, depending on heart rate response [1]. It is necessary to evaluate and monitor for the potential development of ischemic chest pain; and epinephrine may be administered at 2 - 10 µg/min IV or 0.1 - 0.5 µg/kg/min IV, titrated to the desired effect [1, 3]. In this case, the cardiologist decided to administer atropine sulfate for acute management and then proceed with the treatment of choice, either implanting a pacemaker at a cardiac center hospital or monitoring the progression of bradycardia after delivery and scheduling an echocardiography examination. The patient and family chose to pursue regular follow-up at the polyclinic for cardiac monitoring, without pacemaker implantation.
Conclusions
CAVB during pregnancy is rare, with congenital abnormalities being the most common etiology. The method to confirm the diagnosis is through ECG examination. The management of CAVB requires a multidisciplinary approach involving obstetricians, cardiologists and anesthesiologists. Echocardiography evaluation should be conducted after delivery. The choice between vaginal delivery or cesarean section is determined based on obstetric indications. Additionally, patients and families should receive thorough education in determining further management options. The decision to implant a pacemaker depends on the patient’s condition and consent.
Acknowledgments
We would like to thank Prof. Dr. Margono Soekarjo General Hospital, Department of Education and Training, for approving the conduct of this case report. Our gratitude also extends to the medical team and consultants who helped in providing their support throughout the process of writing and publishing this case report.
Financial Disclosure
The authors have not received any financial support for the research, authorship and/or publication for this case report.
Conflict of Interest
The authors confirm that there is no conflict of interest related to the content of this case report.
Informed Consent
Consent was obtained directly from the patient.
Author Contributions
YPD and DMS contributed to the study concept and design. YPD, DMS and AA were in charge of data collection and literature research. DMS and AA were involved in data interpretation and presentation. YPD contributed to the drafting of the manuscript, and WP supervised the research.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
Apgar: activity, pulse, grimace, appearance, respiration; AV: atrioventricular; bpm: beats per minute; CARE: case report guidelines; CAVB: complete atrioventricular block; CO: cardiac output; CPR: cardiopulmonary resuscitation; ECG: electrocardiogram; ESC: European Society of Cardiology; ICU: intensive care unit; IUD: intrauterine device; IV: intravenous; LSCS: lower segment cesarean section; MEC: medical eligibility criteria WHO for contraception; MgSO4: magnesium sulfate; POD: postoperative day; PROM: premature rupture of membranes; SV: stroke volume; SVT: supraventricular tachycardia; SSA/Ro: Sjogren’s syndrome antigen A; SSB/La: Sjogren’s syndrome antigen B; WHO: World Health Organization
| References | ▴Top |
- Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, Goldschlager NF, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(7):e51-e156.
doi pubmed - Swain S, Routray S, Behera S, Mohanty S. Pregnancy with complete heart block. BMJ Case Rep. 2022;15(1):e244598.
doi pubmed - Irianti S, Tjandraprawira KD, Sumawan H, Karwiky G. Total atrioventricular block in pregnancy -Case report. Ann Med Surg (Lond). 2022;75:103441.
doi pubmed - Yafi DA, Noviani C, Saputri RE, Purnawarman A, Andalas Mohd, Yusmalinda Y. Complete heart block in pregnancy: a case report. Indonesian Journal of Cardiology. 2021;42(1):29-34.
doi - Wang K, Xin J, Huang G, Wang X, Yu H. Pregnancy maternal fetal outcomes among pregnancies complicated with atrioventricular block. BMC Pregnancy Childbirth. 2022;22(1):307.
doi pubmed - Viljoen C, Hoevelmann J, Sliwa K, Chin A. Pacing for complete heart block in pregnancy. SA Heart. 2022;19:202-206.
doi - Christensen AP, Singh V, England AJ, Khiani R, Herrey AS. Management and complications of complete heart block in pregnancy. Obstet Med. 2023;16(2):120-122.
doi pubmed - Rihackova E, Vysocanova P, Rihacek M, Kucerova D, Blahovcova T, Kala P. Management of complete heart block in a pregnant woman with systemic lupus erythematosus-associated complications: treatment considerations and pitfalls. Medicina (Kaunas). 2022;59(1):88.
doi pubmed - Hansahiranwadee W. Diagnosis and management of fetal autoimmune atrioventricular block. Int J Womens Health. 2020;12:633-639.
doi pubmed - Carrilho MC, Bravo-Valenzuela NJ, Araujo Junior E. Congenital complete atrioventricular heart block in a pregnant woman with sjogren syndrome: prenatal care follow-up and the challenge of intrauterine treatment. Rev Bras Ginecol Obstet. 2020;42(4):228-232.
doi pubmed - Cunningham FG, Leveno KJ, Dashe JS, Hoffman BL, Spong CY, Casey BM. Williams Obstetrics, 26e. McGraw Hill, 2022.
- Sood R, Kaur H, Mohan G, Nagpal M. Maternal congenital complete heart block in pregnancy: a rare case report. Int J Reprod Contracept Obstet Gynecol 2020;9:3502.
doi - Chegini Zahra, Ali Janati, Asghari-Jafarabadi M, Omid Khosravizadeh. Asymptomatic complete heart block in labor: a case report of none response to atropine therapy. Nursing Practice Today. 2021;8:132-138.
- Wigin C, Hasibuan ER, Soetikno, Yuniadi Y, Wijaya L. Complete atrioventricular block in pregnancy. Indonesia Journal Obstetric Gynecology. 2015;1:56-59.
doi - Saad A, Saade G, Pacheco L. Effect of magnesium sulfate on maternal cardiovascular function in preeclampsia without severe features using non-invasive cardiac monitoring. Am J Obstet Gynecol. 2017;216:S491.
doi - Belfort MA, Saade GR, Moise KJ, Jr. The effect of magnesium sulfate on maternal and fetal blood flow in pregnancy-induced hypertension. Acta Obstet Gynecol Scand. 1993;72(7):526-530.
doi pubmed - Wex F, Luze R, Franke J, Ramoni A, Gizewski E, Lucovnik M, et al. Magnesium sulfate overdose resulting in maternal cardiac arrest: a case report. Clin Obstet Gynecol Reprod Med. 2020;6:1-4.
doi - Sullivan T, Rogalska A, Vargas L. Atrioventricular block in pregnancy: 15.8 seconds of asystole. Cureus. 2020;12(9):e10720.
doi pubmed - Mandal S, Mandal D, Sarkar A, Biswas J, Panja M. Complete heart block and pregnancy outcome: an analysis from Eastern India. SOJ Gynecology, Obstetrics and Women’s Health. 2015;1(1):5.
doi - Paudyal P, Rawal SJ, Khakural P. Challenges in managing pregnancy with complete heart block and its outcome in a tertiary center in Nepal. Journal of SAFOG. 2020;12:359-362.
doi - Hidaka N, Chiba Y, Fukushima K, Wake N. Pregnant women with complete atrioventricular block: perinatal risks and review of management. Pacing Clin Electrophysiol. 2011;34(9):1161-1176.
doi pubmed - European Society of Gynecology, Association for European Paediatric C, German Society for Gender M, Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, Cifkova R, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011;32(24):3147-3197.
doi pubmed - Crea F. Cardiovascular diseases in pregnancy, congenital heart disease, and arrhythmias: lessons from epidemiology. Eur Heart J. 2023;44(9):699-702.
doi pubmed - Young D, Shravan Turaga NS, Amisha FNU, Hayes K, Paydak H, Devabhaktuni SR. Recurrence of complete heart block in pregnancy. HeartRhythm Case Rep. 2021;7(10):679-682.
doi pubmed - Nakashima A, Miyoshi T, Aoki-Kamiya C, Nishio M, Horiuchi C, Tsuritani M, Iwanaga N, et al. Predicting postpartum cardiac events in pregnant women with complete atrioventricular block. J Cardiol. 2019;74(4):347-352.
doi pubmed - World Health Organization. Medical eligibility criteria for contraceptive use, 5th ed. 2015.
- Enevoldsen FC, Nielsen JC, Rasmussen TB. Reversible complete heart block in a pregnant woman related to sertraline treatment. CJC Open. 2022;4(2):240-242.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.