| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 000, Number 000, December 2024, pages 000-000
Placental Pathways: A Case Report on Buprenorphine-Naloxone for Opioid Use Disorder and Placental Pathology
Norah Fanninga, b, Steven Atera, Vickie Melloa
aCentral Michigan University College of Medicine, Mount Pleasant, MI 48602, USA
bCorresponding Author: Norah Fanning, Central Michigan University College of Medicine, Mount Pleasant, MI, USA
Manuscript submitted September 22, 2024, accepted November 26, 2024, published online December 21, 2024
Short title: Buprenorphine-Naloxone for OUD and Placental Pathology
doi: https://doi.org/10.14740/jcgo998
| Abstract | ▴Top |
Opioid use disorder (OUD) during pregnancy is a growing public health concern, and the use of medications for opioid use disorder (MOUD), such as buprenorphine, has become standard for managing OUD to improve maternal and neonatal outcomes. We present the case of a 34-year-old gravida six, para five woman at 38 weeks and 3 days of gestation, treated with buprenorphine, who was admitted for labor induction due to preeclampsia without severe features. The patient had a history of polysubstance use disorder in remission, psychiatric comorbidities, and known placental anomalies, including a two-vessel cord, velamentous cord insertion, and accessory lobe. Following an uncomplicated vaginal delivery, the patient experienced postpartum hemorrhage due to retained placental tissue, requiring medical management and blood transfusion. Pathological examination of the placenta revealed abnormalities consistent with prior literature on opioid exposure during pregnancy. This case is unique in that it highlights the histopathologic impact of long-term opioid exposure and MOUD on placental health, including macroscopic findings of abnormal cord insertion and accessory lobe. We then discuss current literature related to MOUD and placental pathology. Learning points from this case include the need for heightened clinical vigilance in pregnancies involving OUD, MOUD, and the potential effects on placental morphology.
Keywords: Opioid use disorder; Medications for opioid use disorder; Placental pathology; Placental function; Pregnancy; Substance use in pregnancy
| Introduction | ▴Top |
Opioid use disorder (OUD) in pregnancy is a rising public health dilemma in the United States. The Center for Disease Control (CDC) reports that between 1999 and 2014, the prevalence of OUD quadrupled from 1.5 to 6.5 cases per 1,000 delivery hospitalizations [1]. Additionally, the number of women with opioid-related diagnoses at delivery increased by 131% between 2010 and 2017 [2]. Many of these patients are receiving treatment with medications for opioid use disorder (MOUD) that use longer-acting opioid drugs such as methadone or buprenorphine. MOUD has been shown to reduce the risk of relapse and obstetric complications while improving adherence to prenatal care [3, 4]. However, the impact of both opioid use and MOUD on placental function and morphology remains an area of ongoing investigation [5-7].
Previous studies have demonstrated that opioid exposure during pregnancy may be associated with adverse placental outcomes, such as placental insufficiency, increased risk of abruption, and changes in placental morphology [5-7]. Despite these findings, the specific effects of long-acting opioid agonists on the placenta are not fully understood, and this is an evolving area of research. A growing body of small cohort studies, reviews, and case reports has emerged to fill this gap [8-10].
This case report presents unique placental pathology seen in a mother taking buprenorphine-naloxone for OUD during pregnancy, as well as a review of current literature about the effects of opioids on the placenta. This case contributes to the growing body of literature by highlighting the impact of opioids from histopathology to macroscopic levels. Through histopathological examination and clinical correlation, this case offers new insights into the complex interactions between opioid use, MOUD, and placental health, underscoring the need for further research in this area.
| Case Report | ▴Top |
Investigations
This is a case report about a patient being treated with buprenorphine-naloxone for OUD who presented with unique placental anomalies.
The patient was a 34-year-old female, gravida six, para five, living four, who presented to labor and delivery floor triage at 38 weeks 3 days of gestation, with complaints of regular and intense contractions. She had no other associated symptoms. Her past medical history included seizure disorder, anxiety, depression, schizophrenia, and polysubstance use disorder, in remission. Her medications on admission included gabapentin 400 mg three times daily, levetiracetam 500 mg twice daily, and buprenorphine HCl 8 mg-Naloxone HCl 2 mg, also known as Zubsolv, three times daily. The patient endorsed that she used heroin, fentanyl, and benzodiazepines prior to remission. A urinary drug screen in early pregnancy was positive for amphetamines, methamphetamine, benzodiazepines, and buprenorphine. Additionally, she had a history of vacuum-assisted vaginal delivery and manual placental extraction due to retained placenta and postpartum hemorrhage with her fifth gestation. All births occurred vaginally, and patient denied any surgical history.
Diagnosis
Her antenatal course was complicated by known placental anomalies, including a two-vessel cord, accessory lobe, and velamentous cord insertion identified on prenatal ultrasound. No other placental or fetal anomalies were detected on prenatal ultrasound.
Treatment
On admission, she was vitally stable, besides an elevated blood pressure of 151/77 mm Hg. The patient had a history of intermittently elevated blood pressures at outpatient appointments after 20 weeks, including readings above 140/70 mm Hg on two separate occasions. Due to these blood pressures, labs to assess for possible preeclampsia were ordered and identified a protein to creatinine ratio of 0.6, qualifying for an additional diagnosis of preeclampsia without severe features. The other lab work conducted on admission revealed anemia with a hemoglobin of 8.8, hematocrit of 26.7, urinalysis revealing bacteriuria, and positive group B Streptococcus DNA. The urinary drug screen on admission was negative. A cervical check revealed that the patient was 1 cm dilated, 50% effaced, and fetus at -3 station.
Given the new diagnosis of preeclampsia without severe features, a decision was made to begin induction of labor. The induction was undertaken with a Cook catheter, one dose of vaginal Cytotec, artificial rupture of membranes, and Pitocin, resulting in a spontaneous vaginal delivery of a live-born singleton vigorous infant under no anesthesia. The placenta was delivered spontaneously 14 min later. On initial examination, the placenta appeared intact with areas of thinned cotyledons and a velamentous cord attachment. The known history of two-vessel cord was confirmed (Fig. 1a, b). Postpartum oxytocin was started. Blood loss from delivery was estimated to be 350 mL. However, 23 min after delivery of the placenta, the patient was experiencing increasing vaginal bleeding. At that time, bedside ultrasound revealed thin endometrial stripe with single hypoechoic area with the adjacent vascularity near lower uterine segment. The fundus was firm. A bimanual exam was performed, revealing a large clot and single piece of placenta tissue with rough borders. This tissue was extracted and sent to pathology along with the placenta. The patient was given tranexamic acid and Ancef at this time and received a single unit of packed red blood cells later that day. Total estimated blood loss amounted to 2,600 mL.
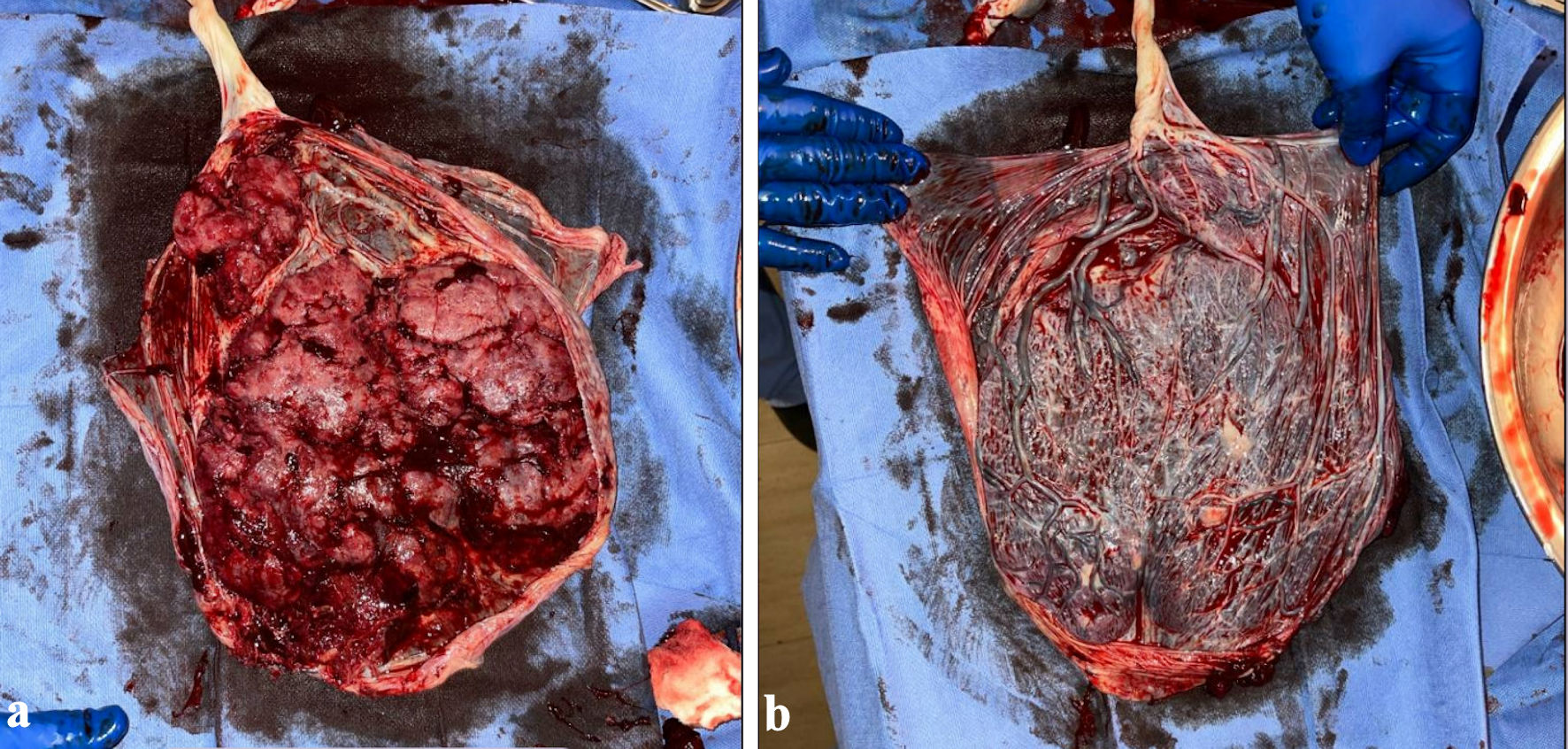 Click for large image | Figure 1. (a) Placenta, maternal side. (b) Placenta, fetal side. |
When the placenta was examined by pathology, gross examination revealed a 500 g, 23 × 16 × 2 cm intact, singleton, ovoid-shaped placenta. The attached extraplacental membranes were pink-tan to purple, thin, semitranslucent with a marginal insertion. The attached, bivascular umbilical cord was 25 cm in length and varied from 1 to 1.5 cm in diameter with moderate dilation along the distal aspect. The umbilical cord had the previously mentioned velamentous insertion with numerous intact, intramembranous blood vessels. The fetal surface was trabeculated, partially nodular and had a moderate amount of subchorionic fibrin deposition. The nodules did not scrape away with ease. The maternal surface was found to be bright red-pink, spongy and had partially disrupted, complete cotyledons. The cotyledons were found to be disrupted adjacent to the velamentous cord insertion. The portion of avulsed placental parenchyma had an associated blood cloth with lines of Zahn without organization and no fibroblasts. There were chorionic villi with third-trimester maturation, focal chorangiosis, patches of intervillous edema and an intervillous hematoma with recent and remote features, occupying less than 5% of total placental parenchymal volume. Many of these histopathologic findings were consistent with placental changes expected in opioid exposure, as we will discuss below.
Follow-up and outcomes
The remainder of the patient’s hospital course was unremarkable, and she remained normotensive with no tachycardia. She was discharged in stable condition with a hemoglobin of 8.1. Her infant was admitted to the neonatal intensive care unit (NICU) and monitored for opioid withdrawal using the neonatal opioid withdrawal syndrome (NOWS) scoring system. The infant developed no signs or symptoms of withdrawal and required no treatment for withdrawal.
| Discussion | ▴Top |
This case study provides insights into the effects of opioid use and MOUD on placental morphology and function in pregnant women with OUD. The findings in this case align with existing literature that describes the potential impact of opioid use and MOUD on placental health. Beginning at a cellular level, it has been suggested that chronic opioid exposure may desensitize or downregulate the placenta’s natural opioid receptors present on trophoblast cells [10]. These opioid receptors are hypothesized to play a physiological role in regulating the immune, vascular, and endocrine functions of the placenta [11]. One proposed pathway is mu opioid receptor stimulation, which has been found to result in nitric oxide (NO) release in human placentas ex vivo [11]. This NO plays a role not only in vascular development of the placenta, but also in the maturation of cerebral blood flow regulation, as well as the development of neuronal activity [6, 12, 13]. Though these studies have been ex vivo or preclinical, researchers have hypothesized that chronic opioid use in pregnancy may induce desensitization and subsequent dysfunction of these placental opioid receptors, which normally function to regulate and facilitate key processes in fetal brain and vascular development [6].
Prior studies found significantly increased incidence of placental and cord abnormalities among patients using heroin compared to non-users. Examples include increased fibrin (clotting) protein depositions, evidence of significant neovascularization, and trophoblastic proliferation and budding. These findings suggest chronic hypoxia and poor vascular integrity [6, 14]. Oxycodone exposure in pregnant mice has also been found to be associated with a reduced area of trophoblast giant cells and a general decrease in the maternal blood vessel area within the labyrinth region of the placenta [7]. One study from 2017 specifically examining placentas from women unexposed or exposed to opioid maintenance therapy with buprenorphine or methadone found that chorangiosis was more common in placentas of the methadone-exposed group versus the unexposed group. Interestingly, there was no statistically significant difference in the odds of this placental lesion in the buprenorphine-exposed group verses the unexposed group [15]. Another study found that delayed villous maturation was more common in the placentas of women exposed to opioid maintenance therapy, and this effect was significantly greater for women treated with methadone and not significant for women treated with buprenorphine [16]. These results suggest that chronic opioid exposure may lead to reduced area of trophoblast giant cells, reduced maternal blood vessel area, neovascularization, and chronic hypoxia [6].
When considering the downstream effects of these placental changes, especially on neonatal outcomes, studies have found that prescription opioid exposure during pregnancy is associated with an increased risk of placental abruption, especially with exposure in both early and late pregnancy [5]. Other studies have found that prenatal opioid exposure significantly increases the risk for fetal growth restriction and preterm labor [17]. In the case presented here, it should also be noted that velamentous cord insertion itself is associated with an increased risk of adverse perinatal outcomes, such as intrauterine fetal demise, small for gestational age, preterm delivery < 37 weeks, and need for manual removal of placenta [18]. Given the cellular, histologic, and morphologic changes discussed above, it can be hypothesized that this chronic opioid exposure has effects at each level that can contribute to these pregnancy outcomes. Despite the evidence discussed, it should also be noted that the patient population being treated with medications for substance use disorder are generally medically complex and have many confounding factors that can complicate the study and treatment of such pregnancies. For example, those patients with OUD are more likely to be current smokers, which is associated with adverse fetal outcomes [19]. Even in the case study presented here, the patient’s medications of gabapentin and levetiracetam and a urine drug screen positive for multiple substances early in substance complicate the interpretation of her placental findings. In that way, this case additionally highlights the difficulty in studying OUD in pregnancy without confounding factors.
Despite the placental changes discussed here, the benefits of treatment with medications of OUD during pregnancy far outweigh the risk of illicit opioids during pregnancy and improve the maternal and neonatal outcomes. Opioid agonist pharmacotherapy prevents opioid withdrawal symptoms and is shown to prevent complications of nonmedical opioid use by reducing relapse risk and its associated consequences. It also improves adherence to prenatal care and addiction treatment programs. Opioid agonist pharmacotherapy in combination with prenatal care has been demonstrated to reduce the risk of obstetric complications [4, 20].
Here, we presented a unique case of placental abnormalities found in a patient receiving treatment for long-term OUD. The patient was being treated with buprenorphine-naloxone and was found to have a two-vessel cord, velamentous cord insertion, accessory lobe, and retained placenta. Her placenta’s histopathology demonstrated a moderate amount of subchorionic fibrin deposition, focal chorangiosis, patches of intervillous edema and an intervillous hematoma, all changes suggesting consistent with chronic hypoxia and poor vascular integrity. This case emphasizes the importance of close monitoring and individualized management of pregnancies complicated by OUD and MOUD. Prompt manual extraction of retained placenta was crucial in achieving favorable maternal and neonatal outcomes, despite the presence of significant placental pathology.
Learning points
This case highlights the importance of close monitoring and individualized care for pregnant patients receiving MOUD. The findings and subsequent literature review emphasize that opioid exposure and MOUD may have significant effects on placental morphology. Despite these placental abnormalities, prompt and appropriate interventions, such as the manual placental extraction performed in this case, can result in favorable maternal and neonatal outcomes. This case underscores the need for further research into the effects of opioid use and MOUD on placental health to optimize care and improve pregnancy outcomes in this population.
Acknowledgments
The authors would like to thank the patient in this case study for her participation.
Financial Disclosure
The authors have no financial disclosures.
Conflict of Interest
There is no conflict of interest for any author.
Informed Consent
Informed consent was obtained from the patient being discussed in this case.
Author Contributions
Norah Fanning contributed to writing, editing, and submission. Steven Ater, DO, contributed to editing and project oversight. Vickie Mello, DO, contributed to editing and project oversight.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
CDC, Center for Disease Control; MOUD, medications for opioid use disorder; OUD, opioid use disorder
| References | ▴Top |
- Miele K, Kim SY, Jones R, Rembert JH, Wachman EM, Shrestha H, Henninger ML, et al. Medication for opioid use disorder during pregnancy - maternal and infant network to understand outcomes associated with use of medication for opioid use disorder during pregnancy (MAT-LINK), 2014-2021. MMWR Surveill Summ. 2023;72(3):1-14.
doi pubmed - Hirai AH, Ko JY, Owens PL, Stocks C, Patrick SW. Neonatal abstinence syndrome and maternal opioid-related diagnoses in the US, 2010-2017. JAMA. 2021;325(2):146-155.
doi pubmed - Opioid Use and Opioid Use Disorder in Pregnancy. Accessed August 30, 2024. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/08/opioid-use-and-opioid-use-disorder-in-pregnancy.
- Jones HE, Martin PR, Heil SH, Kaltenbach K, Selby P, Coyle MG, Stine SM, et al. Treatment of opioid-dependent pregnant women: clinical and research issues. J Subst Abuse Treat. 2008;35(3):245-259.
doi pubmed - Esposito DB, Bateman B, Werler M, Straub L, Mogun H, Hernandez-Diaz S, Huybrechts K. Ischemic placental disease, preterm delivery, and their association with opioid use during pregnancy. Am J Epidemiol. 2022;191(5):759-768.
doi pubmed - Humphries A, Simcox K, Howell B. A review of the literature: How does prenatal opioid exposure impact placental health and fetal brain development? Dev Psychobiol. 2023;65(3):e22378.
doi pubmed - Green MT, Martin RE, Kinkade JA, Schmidt RR, Bivens NJ, Tuteja G, Mao J, et al. Maternal oxycodone treatment causes pathophysiological changes in the mouse placenta. Placenta. 2020;100:96-110.
doi pubmed - Ortigosa S, Friguls B, Joya X, Martinez S, Marinoso ML, Alameda F, Vall O, et al. Feto-placental morphological effects of prenatal exposure to drugs of abuse. Reprod Toxicol. 2012;34(1):73-79.
doi pubmed - Staszewski C, Herrera KM, Kertowidjojo E, Ly V, Iovino N, Garretto D, Kaplan C, et al. Histological changes observed in placentas exposed to medication-assisted treatment. J Pregnancy. 2021;2021:2175026.
doi pubmed - Rosenfeld CS. The placenta as a target of opioid drugsdagger. Biol Reprod. 2022;106(4):676-686.
doi pubmed - Mantione KJ, Angert RM, Cadet P, Kream RM, Stefano GB. Identification of a micro opiate receptor signaling mechanism in human placenta. Med Sci Monit. 2010;16(11):BR347-352.
pubmed - Krause BJ, Hanson MA, Casanello P. Role of nitric oxide in placental vascular development and function. Placenta. 2011;32(11):797-805.
doi pubmed - Northington FJ, Tobin JR, Harris AP, Traystman RJ, Koehler RC. Developmental and regional differences in nitric oxide synthase activity and blood flow in the sheep brain. J Cereb Blood Flow Metab. 1997;17(1):109-115.
doi pubmed - Vavrinkova B, Binder T, Vitkova I, Zivny J. [Placental and umbilical cord changes in drug-addicted women]. Ceska Gynekol. 2001;66(5):345-349.
pubmed - Mokhtari N, Lemon LS, Serra AE, Caritis SN. 804: Chorangiosis in placentas exposed to opioid maintenance therapy. American Journal of Obstetrics and Gynecology. 2017;216(1):S461-S462.
doi - Serra AE, Lemon LS, Mokhtari NB, Parks WT, Catov JM, Venkataramanan R, Caritis SN. Delayed villous maturation in term placentas exposed to opioid maintenance therapy: a retrospective cohort study. Am J Obstet Gynecol. 2017;216(4):418.e1-e5.
doi pubmed - Azuine RE, Ji Y, Chang HY, Kim Y, Ji H, DiBari J, Hong X, et al. Prenatal risk factors and perinatal and postnatal outcomes associated with maternal opioid exposure in an urban, low-income, multiethnic US population. JAMA Netw Open. 2019;2(6):e196405.
doi pubmed - Esakoff TF, Cheng YW, Snowden J, Tran SH, Shaffer BL, Caughey AB. 32: Velamentous cord insertion: does it affect perinatal outcomes? American Journal of Obstetrics & Gynecology. 2012;206(1):S21.
- Cheatle MD, Falcone M, Dhingra L, Lerman C. Independent association of tobacco use with opioid use disorder in patients of European ancestry with chronic non-cancer pain. Drug Alcohol Depend. 2020;209:107901.
doi pubmed - Center for Substance Abuse Treatment. Medication-assisted treatment for opioid addiction during pregnancy. In: Medication-assisted treatment for opioid addiction in opioid treatment programs. Treatment Improvement Protocol (TIP) Series, No. 43. Rockville (MD): Substance Abuse and Mental Health Services Administration; 2005. p. 211-224.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.